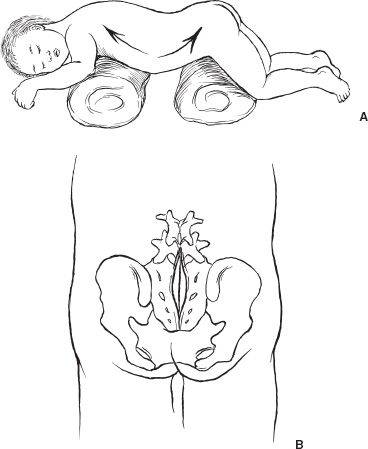
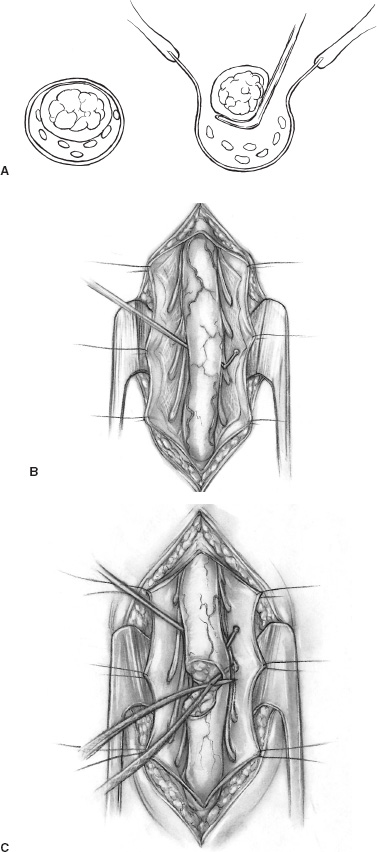
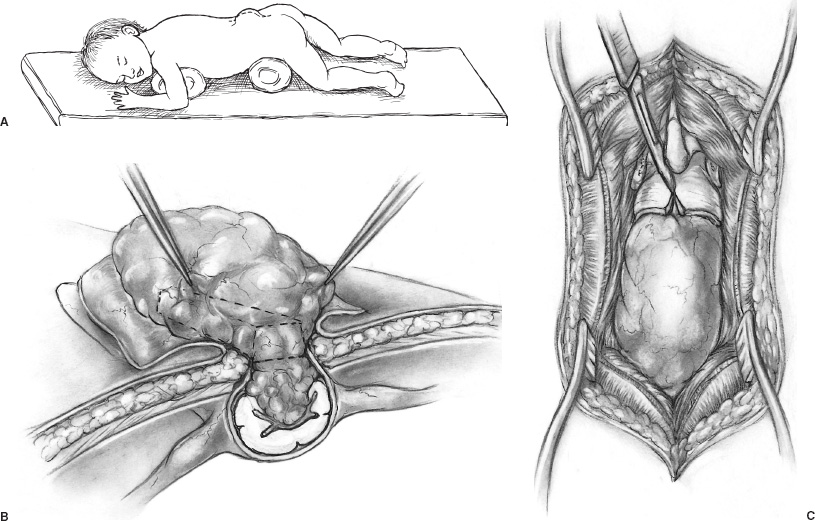
Spina bifida occulta (SBO) is a complex of congenital disorders of the spine that are united by a common etiology and embryologic error, clinical presentation, and occurrence of more than one expression of pathology in a single patient. The seven pathological entities include the tethered spinal cord (TSC) from a thickened, fatty infiltrated filum terminale; lipomyelomeningocele; dermal sinus tract and dermoid; split cord anomaly (diastematomyelia); terminal syrinx; neurentic cyst; and meningocele manqué. A patient frequently expresses more than one pathological manifestation of disease, such as a lipomyelomeningocele coexisting with a TSC and a terminal syrinx; in addition, these problems may coexist with a myelomeningocele. In this case, the filum terminale may be a significant point of fixation below a myelomeningocele, or a myelomeningocele may occur on only one hemicord (hemimyelia) at the site of a split cord anomaly. Without the understanding that lesions are frequently seen together, incomplete or partial operations may be done, and the patient may continue to express the natural history of the unoperated portion of the pathology. The purpose of all operations in this group of conditions is to release points of fixation of the neural tissue and to remove compressing masses. The points of fixation may be caudal, as from a thickened filum terminale; dorsal, as from meningocele manqué; or, occasionally, ventral at the site of a split cord anomaly or a neurentic cyst. The indications for operation on almost all these lesions are simply their presence. Little controversy exists regarding the prophylactic removal of dermal sinuses or dermoids, split cord anomalies, TSC from a thickened filum terminale, or neurentic cysts. Meningocele manqué is generally diagnosed accompanying some other process and may be difficult to confirm with imaging. Terminal syringomyelia, which expands the spinal cord, is generally addressed at the onset of symptoms or before a significant deficit occurs. The only real controversy surrounds lipomyelomeningoceles. A number of well-respected authors (primarily from Europe) advocate a conservative approach until and unless a progressive neurologic deficit develops. This is primarily not a question regarding the natural history but rather concern regarding the risk of an extensive procedure on a complex lesion in an asymptomatic infant. Therefore, justification for taking on the surgical risks rests in the experience of the surgeon. Some surgeons would allow the magnetic resonance (MR) imaging appearance of the lesion to influence operability. In the United States, much less controversy exists, and lipomyelomeningoceles usually are recommended for operation at the time of diagnosis. Prior to operation, patients require a detailed assessment of their neurologic function and the anatomy of their lesion. In small infants, this examination may be challenging, but indirect findings can be seen readily. Loss of muscle bulk and unusual postures of a joint imply neurologic weakness. Seeing the extremity in use may be as much as can be expected in a 6-month-old infant. Some objective measures of neurologic function may be helpful, such as electromyography (EMG) or urodynamics. If a urologic deficit is suspected, documentation of the pre-operative situation may be important in assessing a postoperative deficit. Simply assessing the rectal wink reflex and obtaining a history of a strong urinary stream are inadequate to assess bladder function. I personally rarely use EMG to assess motor function but rely heavily on urodynamics. Routine radiographs of the involved area, ultrasound in young infants, and MR imaging to determine the anatomy of the lesion when the acoustic window is inadequate are routinely performed. On occasion, the ultrasound or MR imaging may be difficult to interpret and computerized tomographic (CT) myelography may be helpful. There are few clear reasons not to recommend operative intervention. In the case of serious life-threatening associated congenital anomalies, where survival is doubtful, surgery to prevent neurologic deterioration is not logical. Even in the setting of significant intellectual debility, maintaining useful lower extremities and bladder control and minimizing neurogenic pain are powerful reasons to proceed with surgery. The timing of operation is a different consideration. If the infant is medically stable and the natural history of the lesion is progression, albeit at an unpredictable rate, I see little argument for postponing intervention and usually proceed within a few weeks to days of diagnosis. All procedures are performed with the patient prone and bolsters placed under the iliac crest and chest to allow free abdominal excursion (Fig. 8–1). Intraoperative monitoring with somatosensory evoked potentials (SSEPs) or EMG can give the surgeon some reassurance but are not used in our clinic. Section of the filum terminale for the TSC should be done in the cul-de-sac of the sacral subarachnoid space. This requires a midline incision from the L-5 spinous process to the midsacrum and a laminectomy of S-1 and S-2 (Fig. 8–1B). The fat infiltrated filum frequently can be seen through the dura. The periostium in small children will appear as a separate layer under the lamina. The inexperienced surgeon might assume this to be the dura and, on incising this structure, confuse it with the epidural fat. The dura is opened in the midline, and the filum is separated from the exiting nerve roots. The filum can be identified as a midline structure with a characteristic vessel on its under surface. It usually exits the dura dorsally in the midline at the point of termination of the sub-arachnoid space. Most importantly, nerve roots have a predictable striation pattern or banding that occurs approximately every millimeter. This pathognomonic finding is absent with the filum. Almost every pathological filum will have fat within it; however, on occasion, fat also may appear within the roots. Following the structures to their point of exiting the dura will add confidence, with neural elements coursing ventral and lateral as opposed to the dorsal midline filum. Neural structures are separated from the filum (Fig. 8–2A), which is coagulated and cut. The sectioning is done first distally and then, a second time, cephalad to the first to allow a specimen to be taken and sent for pathological examination. This examination will confirm the structure sectioned and, with a section removed, the likelihood of the two ends reuniting is remote. All this is done with little or no blood contamination of the subarachnoid space to minimize postoperative adhesions. FIGURE 8–1. A: Position of infant ready to undergo spinal exploration. Bolsters placed under the iliac crest and chest allow free abdominal excursion. B: Position of the midline sacral incision in its relationship to the underlying spine. When the filum is more robust and occupies much of the intradural space (Fig. 8–2B), care must be exercised to section the filum below the last exiting nerve root. The filum must be rotated from side to side to visualize the undersurface and to ensure that no roots remain adherent (Fig. 8–2C). The dura and soft tissues are closed in the usual manner. Alternative techniques include replacing the S-1 and S-2 laminae and visualizing the filum endoscopically. FIGURE 8–2. A: Cross-sectional view of the intradural contents of the sacrum. The large dorsally positioned and fat infiltrated filum is being separated from the adjacent roots. B: Intraoperative view of the sacral intradural contents. The nerve hook is separating the thickened filum prior to sectioning. C: Same view as (B), with the distal filum being sectioned. Lipomyelomeningoceles have two pathological aspects: the fixation of the spinal cord and the mass effect of the fat. The fixation occurs caudally from a thickened filum and either caudally or dorsally at the point the fat exits the dura; this is the primary pathological process. The fat coming into the caudally situated spinal cord will need to be debulked to allow visualization of all points of fixation, but it should not be considered a neoplasm in which the goal of surgery is to remove all neoplastic tissue. Leaving small amounts of fat adherent to nervous tissue is quite acceptable and in fact desirable. The goal of removal of all the fat is unnecessary and dangerous to the continued function of the nervous elements. The patient is positioned prone, as with all other procedures (Fig. 8–3A). A midline skin incision is made over the subcutaneous fatty mass. Skin flaps are elevated off the mass and reflected laterally. The fatty mass is dissected circumferentially from the lumbodorsal fascia. As the edge of the mass is lifted and freed, the neck of the mass protruding through the fascial defect can be seen. All adhesions between the neck and the edge of the fascia should be opened. Redundant fat is removed from the subcutaneous mass (Fig. 8–3B). The fascia cephalad to the neck of the lesion is opened in the midline, and the paraspinal muscle is dissected off the most distal intact spinous process and laminae. Frequently, caudal to the bone is a band of tissue constricting and compressing the upper aspect of the dural neck (Fig. 8–3). The band should be cut and a segment of bone cleared to expose dura within the spinal canal. With the soft tissue reflected laterally, the exposed dura is opened in the midline above the lesion. The spinal cord and its junction with the fat are inspected. Beginning laterally, the dura is opened at its junction with the fat neural complex; this is the most important aspect of a successful procedure. If visualization of this junction is obscured by bone, the bone is removed. If it is obscured by redundant fat, the fat must be debulked, which can be done with a defocused CO2 laser, a routine microsurgical technique, or (as I prefer) with an ultrasonic aspirator. Great care must be exercised in this maneuver to avoid neural injury (Fig. 8–4A). The roots here leave the cord in an unpredictable fashion coursing caudally, horizontally, or even cephalad. They may lose the regular segmented orientation and be bunched in large groups or have long skip areas. Sensory roots coming from the dorsal aspect of the cord and hugging the inner aspect of the dura are most vulnerable to injury. Slowly, as the junction of the dura with the fat–neural complex is opened, the intradural anatomy is revealed. Frequently, before each cut in the dura, fat will need to be debulked. Routine microsurgical technique is associated with troublesome bleeding, with the cut end of the vessel retracting into the fat. Ultrasonic aspiration tends to preserve these vascular structures, allowing them to be coagulated and cut prospectively. Low suction and power settings are effective with this process.
INDICATIONS AND PREOPERATIVE EVALUATION
Contraindications
INTRAOPERATIVE TECHNIQUE
Tethered Spinal Cord
Lipomyelomeningoceles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


