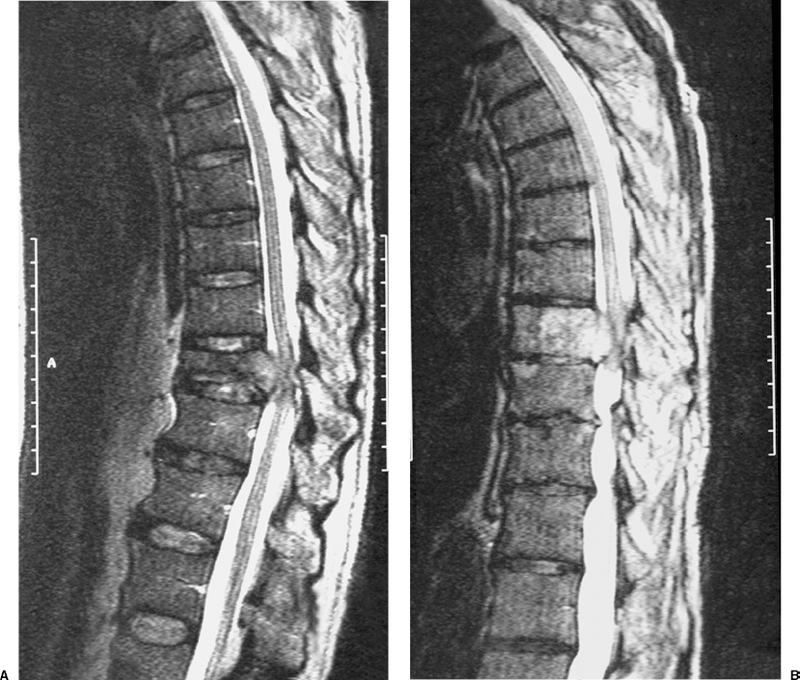
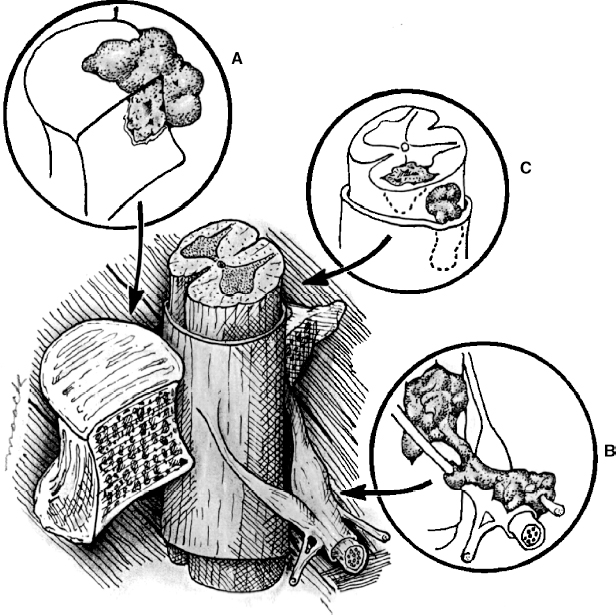
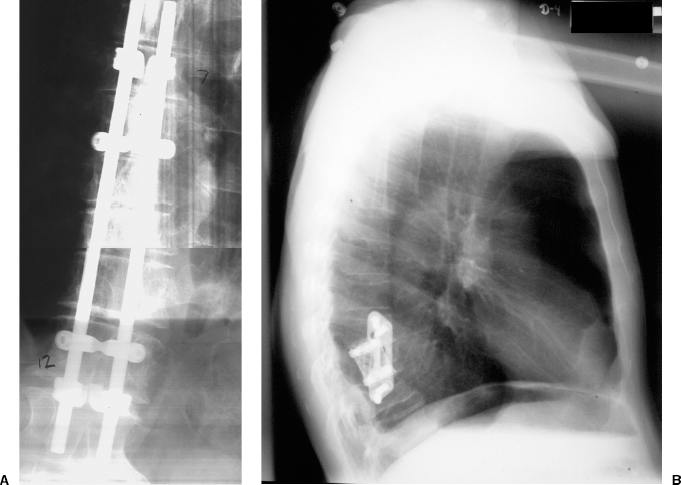
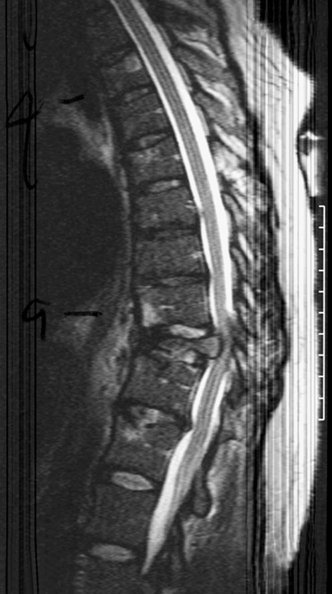
22 Spinal Cord Compression Secondary to Neoplastic Disease: Epidural Metastases and Pathologic Fracture Phillip A. Tibbs and Roy A. Patchell Spinal cord compression frequently presents as a medical emergency with rapidly progressive loss of neurological control of the extremities, bladder, and bowel (Fig. 22-1A,B).1 The patient may or may not have a known diagnosis of cancer. In many cases the onset of paraparesis or quadriparesis from cord compression may be the first evidence of occult malignancy.2 Pain is the universal harbinger of cord compression and, all too often, patients are managed with escalating doses of narcotic analgesics to treat a pain of unknown origin until the expanding tumor causes devastating neurological consequences. For many years, radiotherapy and corticosteroids were the standard of care for patients with spinal metastases and cord compression.3 This was an era prior to accurate radiographic imaging of the spine and prior to advances in neurosurgical technique allowing direct decompression and reconstruction of the affected vertebral segments. New studies, including an extensive meta-analysis of the literature and the first reported prospective randomized trial of radical surgery plus radiotherapy versus radiotherapy alone, have convincingly demonstrated that surgical therapy is the superior modality and the primary treatment of choice in selected patients with metastatic epidural spinal cord compressions (MESCCs).4,5 Neurosurgeons therefore will be consulted more frequently and earlier in the course of management of these patients and must be prepared to advise regarding operability and case selection as well as be knowledgeable about decompressive and surgical reconstruction techniques. Acute onset of neurological deficit requires immediate treatment if optimal clinical outcome with preservation and recovery of neurological functions is to be achieved.1 MESCC is a frequent complication of cancer, occurring in 5% to 14% of cancer patients and causing over 20,000 cases of cord compression per year in the United States.3 When cancer spreads, the spine is its most common target, and up to 40% of cancer patients develop this complication of systemic malignancy, causing debilitating pain even in the absence of paralysis.6 As life expectancies are lengthened due to improvements in cancer care, it is likely that an increasing number of patients will survive long enough to develop MESCC. The osseous vertebral column is affected in 85% of cases, paravertebral sites in 10% to 15%, and there are rare cases of isolated epidural or intramedullary metastasis.7 Figure 22-2 depicts the most common locations of spinal metastases. Approximately 75% of spinal metastases occur in the thoracic spine, 20% in the lumbar spine, and 10% in the cervical spine.8 In 20% to 40% of patients with spinal metastases, multiple noncontiguous sites of involvement can be found.7 Breast, lung, and prostate cancers account for ~50% of spinal metastases.3 The remaining 50% include (in decreasing order of frequency) renal cell carcinoma, gastrointestinal (GI) malignancy, thyroid cancer, lymphoma, and multiple myeloma. Some primary tumor types have a very high incidence of spinal metastasis in the course of the disease, including prostate at 90%, breast at 75%, melanoma at 55%, and lung cancer at 45%.9 Well into the 1990s an extensive body of medical literature has supported the notion that the combination of radiotherapy and corticosteroids (RT+CS) is the initial treatment of choice for MESCC.10,11 Numerous articles showed no benefit of surgery for cord decompression over radiotherapy and steroid administration.8,11,12 In this literature, “surgery” essentially equaled laminectomy. Laminectomy does not allow direct access and decompression for the majority of cases of MESCC where the metastatic deposit is anterior to the cord. Laminectomy not only does not provide adequate surgical exposure to allow reconstruction of the damaged vertebra, it also may destabilize the spine, resecting the only intact column of stability.8,11,13 This treatment standard of RT+CS kept only ~50% of patients ambulatory, and few nonambulatory patients ever regained functional independence.8,11 These reports were retrospective analyses in an era where the quality of radiographic imaging was limited and surgery consisted mostly of decompressive laminectomy. Often the extent of systemic disease and spinal involvement was poorly understood, there was little thought given to the biomechanics of spinal stability, and the revolution of instrumentation for spinal reconstruction was embryonic. Figure 22-1 (A) Midthoracic vertebral metastasis with pathological fracture of bone compressing spinal cord. (B) Upper thoracic vertebral metastasis with collapse of the vertebral body and large epidural tumor mass anterior to the cord. Due to this discouraging literature, there has been a natural reluctance to consider surgery as an option. Neurosurgeons were most commonly consulted when neurological deficit progressed despite radiotherapy, when delayed recurrence developed in a spinal region that had received prior maximum radiation dosage, or when the destruction of the vertebral body had progressed to the point of pathological fracture with obvious instability. In this clinical setting, where surgery is relegated to a salvage role in late-stage cases, high morbidity and poor outcome are expected. A heavily radiated surgical field in a patient on high-dose steroids is a prescription for surgical complications, including wound infection, wound dehiscence, failure of instrumented stabilization, and nonunion. Beginning in the 1980s, with improved cancer staging and spinal imaging by computed tomography (CT) and ultimately magnetic resonance imaging (MRI), the possibility of direct attack on the metastatic tumor was explored by several surgeons.14,15 The fact that in the majority of cases of MESCC the epicenter of the tumor is anterior to the spinal cord necessitated development of surgical teams that include a thoracic or general surgeon to provide anterior access and a neurosurgeon to perform decompression and stabilization. Complex instrumentation systems that were initially conceived to treat scoliosis, vertebral fracture, and degenerative spinal conditions were adapted and improved to allow reduction of cancer-related pathological fracture and correction of spinal instability (Fig. 22-3A,B).16,17 Changing the standard of care for such a complex clinical problem requires more than anecdotal or retrospective reports. Oncologists, radiotherapists, patients, and surgeons themselves have needed objective data to properly direct therapy toward surgery or RT+CS based upon rigorous analysis of the literature and methodologically sound trials. Figure 22-2 (A) Locations of metastases to the spine. Most tumor emboli seed the vertebral column surrounding the spinal cord, with the posterior half of the vertebral body being the most common initial focus. (B) Tumor can also originate in a paravertebral location and track along the spinal nerves to enter the spinal column by way of the neural foramina. Both of these mechanisms can lead to epidural spinal cord compression. (C) Intramedullary, subdural/leptomeningeal, and isolated epidural metastatic deposits are rarely encountered. (From Klimo P Jr, Schmidt MH. Surgical management of spinal metastases. Oncologist 2004;9:188–196.) Klimo et al recently published a detailed meta-analysis of 1542 patients who had undergone either radiation or surgery plus radiation for MESCC.4 Patients treated with surgery plus RT+CS achieved an 85% rate of ambulation (recovery from paraparesis or preservation of ambulation) versus 64% for the patients receiving radiotherapy alone. Citing our work as the first randomized clinical trial to be presented on this subject, they concluded that the option of surgery should be considered as the primary modality in patients with MESCC with conventional radiation postoperatively as adjuvant therapy. The authors have completed a multi-institutional, National Institutes of Health (NIH)-funded, prospective, randomized trial of direct decompressive surgical resection of epidural metastasis followed by radiotherapy versus radiotherapy alone. Our study demonstrates that the combination of surgery plus radiation is superior to radiation alone in preserving intact neurological function, recovering lost ambulatory capacity, preservation of bladder and bowel function, maintaining quality of life, and improving pain control.5 Furthermore, patients initially randomized to radiotherapy who failed treatment and crossed over to surgery had poorer outcomes and more complications than patients receiving surgery as primary therapy. This emphasizes that surgery should be the first treatment in appropriate patients and not used as a salvage procedure.18 Figure 22-3 (A) Midthoracic epidural metastasis localized to the lamina, pedicle, and dorsal cord after decompressive laminectomy, stabilization by a compression rod construct to reconstitute the dorsal tension band. (B) Thoracic corpectomy for metastasis reconstructed with a titanium cage and transvertebral screw and plate fixation. Comprehensive clinical evaluation includes the recording of a thorough and accurate history, an understanding of risk factors particular to each individual patient, a careful neurological and musculoskeletal examination, and review of definitive radiographic imaging. The neurological examination should include a segmental motor examination of the extremities using the Denny-Brown system, evaluation of deep tendon reflexes looking for hyperreflexia or pathological reflexes indicating upper motor neuron involvement, and careful sensory examination to define a discrete sensory level. Assessment of bladder and bowel function can be made through history and the rectal examination. Radiographic evaluation includes enhanced total spinal MRI to determine whether more than one area of vertebral involvement may be responsible for neurological deficit.19 Likewise, imaging the brain is usually worthwhile because a negative study is reassuring, whereas a study positive for brain metastasis may not only explain some of the patient’s neurological deficit but may also impact upon the decision to operate given that the intracranial lesion will limit life expectancy. Anteroposterior (AP) and lateral spine radiographs reveal spinal deformity due to pathological fracture, and high-resolution CT of the spine with sagittal reconstruction often reveals details of bony destruction surpassing MRI and assisting with the decision to surgically stabilize a severely weakened vertebral segment. Although there is increasing evidence that the advanced surgical techniques available today are superior to RT+CS in many cases, not all patients with MESCC are candidates for surgery. It is the responsibility of the consulting neurosurgeon to make a recommendation for surgery based upon sound clinical criteria deriving from the current literature and surgical experience. Key factors in determining whether an individual will potentially benefit from surgery include the following: On confirming the diagnosis of spinal cord compression due to metastatic tumor, the patient is given a loading dose of dexamethasone followed by a maintenance regimen. In our study we administered a 100-mg dose of dexamethasone initially followed by 24 mg q 6 hours.5 These large doses were selected because they appear to be the highest dose known to have therapeutic benefit. Diabetic patients and patients with a history of sensitivity to or adverse affect from high-dose steroids should be given lesser dosages. Appropriate antibiotic prophylaxis is essential. In general, intravenous cephalosporins cover staphylococcus and most other organisms that complicate clean surgical cases.23 If the patient in question has been septic or has known urinary tract infection or other organ system infection, coverage should be extended to cover any identified bacterial organism particularly if instrumentation systems are to be implanted. Preoperative laboratory workup must include coagulation studies and complete blood count. Patients with systemic malignancy are often anemic and should be transfused to a hematocrit of well over 30 prior to beginning a major neurosurgical procedure, and type and cross-matched blood must be immediately available because some metastatic tumors such as renal cell carcinoma and melanoma are notoriously vascular. Likewise cancer patients, especially patients with lymphoreticular malignancy, may require platelet transfusions or fresh frozen plasma administration before it is safe to proceed with surgery. If surgery is the appropriate therapy for a patient with MESCC, time is of the essence. Neurological function may deteriorate rapidly by progressive tumor expansion and suddenly by either pathological collapse of the vertebra or vascular compromise of the cord (Fig. 22-4). Moreover, if the paresis progresses to plegia and a total deficit persists for more than 24 hours, the prognosis is dismal for neurological recovery even with excellent surgical intervention. It is clear therefore that intervention should be expedited and handled as an emergency.10 The decision on timing is tempered by the fact that preoperative preparations must be completed. The team of access surgeon and neurosurgeon must be assembled, and necessary operating room staff and instrumentation systems must be available for the case to begin.
Epidemiology
Evolution of the Standard of Care for Metastatic Epidural Spinal Cord Compression
Clinical Evaluation
Patient Selection for Surgery
Preoperative Care
Surgical Management
Spinal Cord Compression Secondary to Neoplastic Disease: Epidural Metastases and Pathologic Fracture
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








