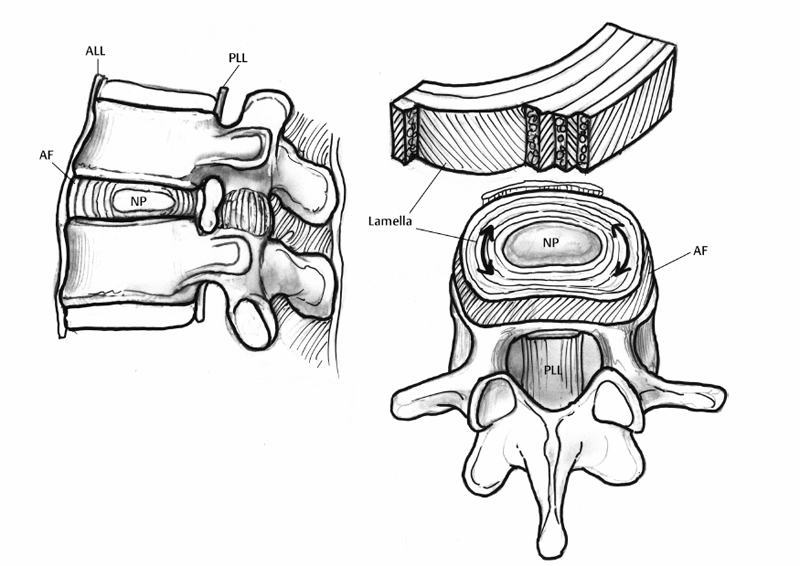
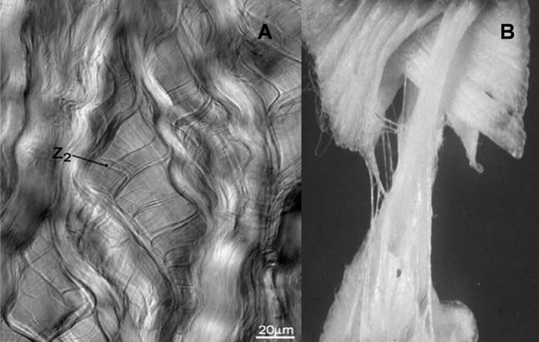
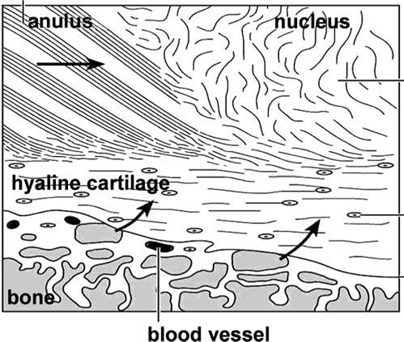
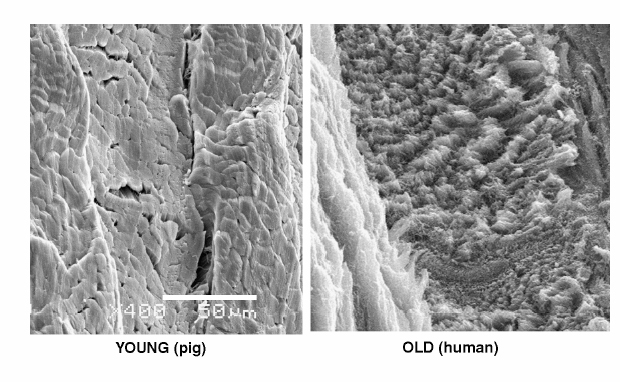
2 Anatomy and Physiology of the Lumbar Intervertebral Disc and Endplates Michael A. Adams Intervertebral discs (IVDs) are pads of fibrocartilage that lie between the vertebral bodies of the spine. Their primary function is to confer some degree of flexibility to the spinal column, while at the same time distributing compressive loading evenly to the vertebral bodies, even when the spine is subjected to complex loading. Discs are generally too stiff and thin to be good shock absorbers, and leave most of this task to the body’s natural shock absorbers—the tendons.1 In the first sections of this chapter, a detailed anatomy of IVDs, from the structure and composition of the extracellular matrix, to the characteristics of disc cells and their metabolite transport is given. Changes during growth and chronologic aging are then considered, to show progressive and inevitable changes in disc anatomy during the lifespan. This sets the scene for the final section on disc degeneration, which considers deleterious changes in structure and function, which occur in some discs but not in others. This discussion leads to a proposal for how disc degeneration should be defined, and a brief overview of causality. The gross anatomy of a human lumbar IVD is illustrated in Fig. 2.1. The soft, pulpy nucleus pulposus is surrounded and constrained by the tough concentric layers (lamellae) of the anulus fibrosus (AF). High water content and low rigidity in the nucleus allow it to behave mechanically like a compressed fluid,2 which generates a tensile “hoop stress” in the anulus when the spine is compressed. However, the mature anulus has sufficient rigidity to resist compressive loading directly,2 as well as to resist nucleus pressure, so it is a complex mechanical structure. Overall disc dimensions vary with spinal level and increase with increasing body height.3 Collagen fiber bundles of the anulus are oriented at ~30 degrees to the horizontal, with the direction alternating between adjacent lamellae (Fig. 2.1); 20 to 60 separate bundles are discernible in a vertical section.4 Adjacent lamellae are of irregular thickness and often are discontinuous, especially in the posterolateral anulus.4 Fibers of the outer anulus are strongly embedded in the adjacent vertebral bodies (forming Sharpey’s fibers). In the inner anulus, they curve around the nucleus and blend in gradually with the hyaline cartilage of the endplate. The collagen bundles of the anulus are mostly composed of coarse collagen type I fibers. They exhibit a planar zigzag “crimp” pattern when viewed under a polarizing microscope (Fig. 2.2). Crimp can be straightened out, allowing collagen type I fibers to be stretched ~4% with little resistance (as measured in tendon).5 Stretching of the anulus also causes individual fibers to reorient and slide relative to each other.6 Collagen fibers in the same lamella split and intertwine with each other laterally (Fig. 2.2) to form a cohesive planar structure.7 Strong interactions between collagen fibers and other matrix constituents (collagen means “glue-maker”) contribute toward structural integrity, even when collagen fibers are disrupted.8 This makes the anulus very tough and difficult to pull apart. Approximately 20% of the collagen in the outer anulus is type II, which forms very fine “fibrils” rather than coarse type I fibers, and the proportion of collagen type II rises to 70% in the inner anulus.9 Adjacent lamellae of the anulus are only weakly bonded together10 probably by fine elastin11 and collagen type II fibrils, with the former conferring the property of “elastic recoil,” which is the ability to spring back into place after large deformations. Other constituents of the anulus include the water-binding proteoglycans, which compose ~10% of the dry weight of the outer anulus and 30% of the inner anulus.9 Excised samples of anulus can be stretched circumferentially by 40 to 60% before gross failure occurs, at a strain (elongation) of 40 to 64% and a stress (force per unit area) of 2 to 7 MPa.12 The inner anulus is weaker and more deformable than the outer anulus12,13; the posterolateral anulus is particularly weak.13 Minor matrix components of both the anulus and nucleus include collagen types III and VI, which reinforce the pericellular matrix.14 Fibronectin helps to bind cells to their matrix and is more abundant (often in fragments) in degenerated discs.15 The nucleus is normally a soft and deformable proteoglycan gel, which is reinforced by fibrous proteins. Proteoglycans are neutral molecules overall but are made up of glycosaminoglycan subunits, which have a preponderance of fixed negative charges on their exterior surface (balanced by positive charges internally). The fixed negative charges attract the positive charges found on either end of the polarized water molecule, thereby attracting water to the glycosaminoglycans. In young adults, proteoglycan molecules constitute 70% of nucleus dry weight,9 and tissue water content can exceed 80%.9 Proteoglycans aggregate with hyaluronic acid to form very large molecules that are trapped within the tissue by the loose network of collagen type II fibrils, which comprise more than 80% of collagen in the nucleus.9 Elastin fibers appear to have a predominantly radial arrangement within the nucleus,16 possibly serving to resist radial migration of nucleus tissue down radial fissures. Under high pressure, nucleus pulposus material can insinuate between collagen fibers of the inner anulus, causing microscopic disruption within and between lamellae.17 Fig. 2.1 An intervertebral disc lies between adjacent vertebral bodies of a spinal motion segment (left). The soft nucleus pulposus (NP) is surrounded by 15 to 25 lamellae (right, top) of the anulus fibrosus (AF). Spinal compression generates a hydrostatic pressure in the NP and a restraining tensile hoop stress in the anulus. The posterior longitudinal ligament (PLL) acts as a “nerve net” for the underlying disc. The anterior longitudinal ligament (ALL) helps to protect the disc from excessive backward bending. Fig. 2.2 (A) Phase-contrast microscopy of branching, crimped collagen fibers in bovine tail anulus fibrosus. (B) Sample of young adult human anulus pulled apart vertically in a tensile test in vitro: note the collagen fibers pulling out of the proteoglycan matrix, and the alternating fiber direction in adjacent lamellae. (A, From Pezowicz CA, Robertson PA, Broom ND. Intralamellar relationships within the collagenous architecture of the annulus fibrosus imaged in its fully hydrated state. J Anat 2005;207(4):299–312. Reprinted with permission. B, From Adams MA, Bogduk N, Burton K, Dolan P. The Biomechanics of Back Pain. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2006. Reprinted with permission.) This thin layer of hyaline cartilage is only weakly bonded to the underlying bony endplate (Fig. 2.3). Like articular cartilage, it has a dense organized network of fine collagen II fibrils, which strongly maintain its structure and shape, prohibit swelling, and make it stiffer and denser than the adjacent nucleus. The cartilage endplate serves to maintain internal pressurization of the disc by hindering the expulsion of water and proteoglycans from the disc through the bony endplate into the vertebral body. It also acts as a biological filter to restrict migration of cells and large solutes between nucleus and bone. The cells are rounded chondrocytes, similar to those in articular cartilage. Fig. 2.3 Detail showing the hyaline cartilage endplate (center) bonded to the disc (top) and the bony endplate (bottom). Arrows show the two routes for metabolite transport into the center of the avascular disc: via the endplate route and via the peripheral anulus. Note how the coarse collagen fibers of the anulus blend in with the hyaline cartilage. (From Adams MA, Bogduk N, Burton K, Dolan P. The Biomechanics of Back Pain. 2nd ed. Edinburgh, UK: Churchill Livingstone; 2006. Reprinted with permission.) This is a thin sheet of bone, ~1 mm thick, which forms part of the cortex of the vertebral body. It contains a radiating anastomosing network of small marrow cavities, especially in the central regions,18 which allow direct contact between bone marrow and hyaline cartilage endplate for ~5 to 10% of the central endplate area.19 This facilitates the transfer of metabolites between blood vessels in the vertebral marrow and cells in the adjacent disc. Cranial endplates are 10 to 15% thinner20 and weaker21 than caudal endplates, and both are thinnest and weakest in their central region. Under normal levels of compressive loading, endplates bulge outward into their adjacent vertebral bodies, typically by 0.25 mm.22 Not surprisingly, compressive fracture of the central region of the cranial endplate is common, both in vivo and in vitro.20 The mechanical vulnerability of the bony endplate is probably a concession to the nutritional demands of the adjacent discs, and the relative vulnerability of the cranial endplate can be explained in terms of the extra loading applied to the caudal half of the vertebral body by muscles attached to the neural arch (and transmitted to the vertebral body at the level of the pedicle). Typical cells of the anulus are elongated fibroblasts, which tend to lie parallel to the lamellae of the matrix, although some anulus cells are more rounded.23 They all appear to have long cytoplasmic extensions,24 which can form gap junctions with extensions from neighboring cells,6 suggesting that anulus cells are capable of forming a “nerve net” comparable to that created by osteocytes in bone. Anulus cells synthesize mostly collagen and proteoglycans. Nucleus cells resemble the rounded chondrocytes found in articular cartilage, and their function is similar: to synthesize the proteoglycans and the fine, collagen type II fibrils of their matrix. Chondrocytes are not present in the nucleus from birth but replace the original notochordal cells during early childhood. Notochordal cells are larger and more active, form clusters, and appear to form gap junctions with each other.25 Their demise after childhood may be attributable to increased mechanical loading, or to increasing difficulties in transporting metabolites into and out of the avascular disc as it grows larger. Probably for similar reasons, disc cell density decreases during growth, but then remains constant for most of adult life.26 Mature nucleus cells retain sizeable cytoplasmic extensions.24 The relative isolation of nucleus cells from the general circulation suggests that autoimmune reactions will occur if nucleus cells escape from the center of the disc, as they do in disc herniation. Recent experiments on young pigs have shown that transplanted autologous nucleus pulposus tissue does indeed attract activated T and B cells to it,27 but it remains to be seen if the same reaction occurs with nucleus cells from middle-aged humans. Cell density in the adult human disc is maximal in the outer anulus,23 close to the peripheral blood supply. It is noteworthy that only the outer anulus is capable of effective healing following injury.28 In the absence of a nerve and blood supply, disc cells communicate by secreting and receiving low-molecular-weight chemical messengers (cytokines), which diffuse mostly to near-neighbor cells before being degraded. To suggest that cytokines (such as interleukin-1) are the “cause” of disc degeneration is rather like confusing the messenger with the message, although it is possible that malfunctioning of the cell-signaling apparatus could predispose a disc to degeneration. In the first few years of life, blood vessels from the vertebral body penetrate the cartilage endplate and extend into the inner and outer anulus, but not into the nucleus.29 These blood vessels are lost during infancy, presumably because weight-bearing causes hollow blood vessels to collapse within cartilage. High concentrations of proteoglycans may also play a role in inhibiting blood vessels from growing into central regions of a healthy disc.30 Normal adolescent and adult discs are avascular, apart from a few capillaries penetrating the peripheral anulus, adjacent to the insertion of ligaments.29 In the absence of a blood supply, disc cells must receive their nutrients by the processes of diffusion and fluid flow. Diffusion involves flow down a concentration gradient, stimulated by random Brownian movements, and is an efficient system for the transport over short distances of small molecules such as glucose and oxygen. Large molecular weight solutes (such as the proteins, which control many cell activities) diffuse only very slowly in the disc and rely on being transported along with the bulk flow of fluid in response to changes in applied mechanical loading. Diurnal cycles of activity and rest cause 20% of disc water to be exchanged every day,31 so fluid flow contributes substantially to metabolite transport,32 especially in the anulus.33 Rapid fluid “pumping” during locomotion probably has little effect, although alternating periods of rest and activity could possibly be beneficial. Disc cell metabolism is well adapted to an environment that is low in oxygen and yet high in the lactic acid that is an endproduct of anaerobic metabolism.34 Furthermore, cell density in the nucleus appears to reflect an adaptation to restricted metabolite transport.35 Although viable, the resulting population of disc cells appears to be too small to effect major repairs,28 or to overcome any disturbance in the already precarious supply of metabolites caused by heavy smoking36 or endplate calcification. Cells are most responsive to the type of loading they normally encounter, so nucleus cells respond to hydrostatic pressure, and outer anulus cells to stretch, but not vice versa. Inner anulus cells experience2 (and respond to) hydrostatic pressure. Moderate mechanical stimuli cause both cell types to synthesize more matrix, whereas low and static stimuli cause them to synthesize less,37 in accordance with the principles of “adaptive remodeling.” High and static loading inhibits disc cell synthesis and can stimulate them to release and activate matrix metalloproteinases, enzymes that break down matrix macromolecules.37,38 Sustained loading influences disc cell metabolism indirectly by increasing or decreasing the supply of metabolites, as discussed above. Furthermore, prolonged loading can greatly dehydrate disc tissue,33 so that reduced pore size (and hence permeability) interferes with diffusion and fluid flow through the matrix. In normal adult human discs, both free and complex nerve endings are confined to the peripheral anulus, typically to the outermost 1 to 3 mm.39 Anteriorly and laterally, the nerves are sympathetic, but in the posterior quadrant of the disc they originate from the mixed sinuvertebral nerve40 and appear to be capable of nocioception.41 Pain provocation studies on sedated humans provide direct evidence that the posterior AF and the adhering posterior longitudinal ligament are the most common tissues of origin of severe and chronic back pain.42 The same study supports the concept of “pain-sensitization” because it shows that patients’ typical pain can be reproduced by relatively gentle mechanical probing of the posterior anulus. Animal experiments have confirmed that mere contact with the autologous nucleus pulposus can alter the morphology and conduction velocity of neurons43 and produce pain-like behavior in rats, which is worsened if the nerve tissue is simultaneously stretched.44 Nerves believed to be capable of nocioception can grow in toward the nucleus in severely degenerated discs, in both the anterior45 and posterior46 anulus. Nerves need to be close to blood vessels, and both structures appear to grow in together along the zone of granulation tissue within radial fissures.46 Nerve ingrowth may be caused by revascularization, which itself is a consequence of reduced pressure and proteoglycan content in the degenerated nucleus. Nerve ingrowth may also be stimulated by neurotrophic factors released by disc cells47 and by a loss of proteoglycans, which have an inhibitory effect on nerve growth.48 Microscopic changes including “mucoid degeneration” occur before the age of 2 years, and this is soon followed by decreased vascularization of the endplate and by decreased disc cellularity.49 These changes may simply reflect necessary adaptations to weight-bearing and growth because they vary little with spinal level49 and appear to have little effect on disc function.2 Macroscopic disc changes before the age of 20 years have been characterized as “fibrous transformation,”50 but their relationship to subsequent pain-related features such as radial fissures and disc prolapse (see below) is not clear because the latter do not normally appear for another 20 years. After skeletal maturity, proteoglycan aggregates become increasingly fragmented, with the greatest changes occurring in the nucleus.9 Some proteoglycan fragments are lost, so water content declines, especially in the nucleus, where tissue hydration falls from 87% to 74% between childhood and old age.9 The collagen network is affected rather differently. During growth, collagen fibers become stiffer and stronger as cross-links between adjacent collagen molecules become more stable.51 From skeletal maturity onward, collagen cross-linking is supplemented by nonenzymatic (hence uncontrolled) cross-linking that involves sugar molecules. The biochemistry is complex, but the result of these adventitious chemical reactions is that the collagen network becomes excessively cross-linked, making the matrix stiffer (rather like a delicate woolen garment washed at a high temperature). Figure 2.4 compares collagen fibers in young pig anulus and aging adult human anulus. Experiments on articular cartilage show that this phenomenon of nonenzymatic glycation makes cartilage more vulnerable to damage.52 Nonenzymatic glycation occurs most in those tissues that do not turn over (i.e., replace) their collagen very quickly, so the avascular IVD, with a collagen turnover time of more than 100 years, is greatly affected with advancing age.51 Nonenzymatic glycation causes collagenous tissue to develop the characteristic yellow-brown coloration typical of aging.
Anatomy
Anulus Fibrosus
Nucleus Pulposus
Cartilage Endplate
Bony Endplate
Physiology
Disc Metabolite Transport
Mechanical Influences on Disc Metabolism
Disc Innervation and Discogenic Pain
Effect of Aging on Anatomy and Physiology
Changes in the Extracellular Matrix
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








