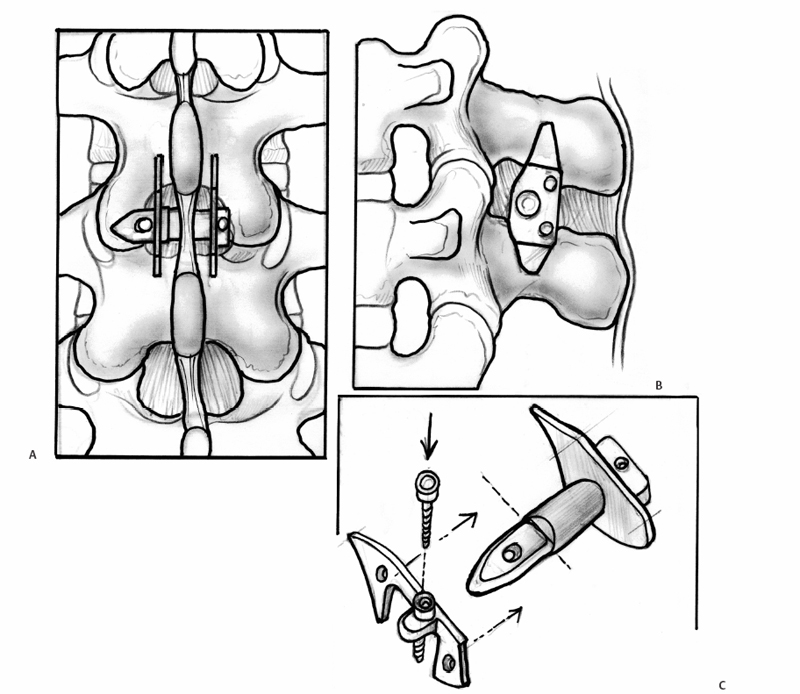
23 Interspinous Devices Mario L. Pereira, Stephen H. Hochschuler, and Donna D. Ohnmeiss The management of painful disc degeneration remains controversial, with traditional surgical treatment for painful degeneration being fusion. Ideally, interbody fusion could address the disc as the pain generator. Total disc replacements have been introduced as an optional alternative. These devices are designed to relieve pain and allow motion of the diseased segment. Given that narrowing of the foramen and mechanical loading of the facet joints play a role in degenerative back pain, devices that distract the foramen, unload the disc, and retain motion are appealing. In this chapter, we will review the various interspinous implants and the biomechanical and clinical findings to date, as well as issues related to their potential application in the treatment of painful disc degeneration. It has long been accepted that biomechanical load has a detrimental effect on the intervertebral disc. In animal studies, it has been described that following compression across the disc, distraction resulted in restoration of the disc.1,2 Based on this finding and biomechanical studies suggesting that interspinous devices may unload the disc, there has been interest in using these devices to treat painful disc degeneration. However, there is very little information on this particular application. Below is an overview of the biomechanics of interspinous implants and the various types of interspinous devices available to date. Most of the investigation of these devices has dealt with their potential role in the treatment of spinal stenosis. In an attempt to counter the detrimental effects of degenerative spinal conditions, primarily stenosis and disc degeneration, interspinous implants were developed. These devices are spacers between the spinous processes that limit extension at the symptomatic level, having little or no effect on rotation, side bending, or flexion of the lumbar spine. Implantation of an interspinous device is a minimally invasive procedure that may be implanted with MAC sedation and local anesthesia, resulting in a relatively quicker surgical recovery. A current limiting factor is that the design typically limits their use to levels above L5-S1. The two primary therapeutic biomechanical changes associated with the use of interspinous devices are distraction of the neuroforamen and unloading of the intervertebral disc. These devices dissipate forces across the posterior spinal elements including the facet joints.3–5 Biomechanical testing has found that interspinous devices do not affect the segments adjacent to the implant with respect to intradiscal pressure; motion;5,6 neuroforaminal height, width, and area;7,8 or facet loading.4 Cadaveric studies suggest that a reduction in disc pressure at the posterior endplates does occur at the implanted segment.5,6 Therefore, the effects of interspinous devices tend to be a local phenomenon, without any ill effects on the adjacent segments.9 In a study using finite modeling, it was reported that implant forces were significantly impacted by the height of the implant, but not by stiffness of the device.10 The size and stiffness of the device had only minimal effect on the disc pressure. However, the stresses in the vertebral arch were significantly increased. This relates to a potential concern with interspinous devices — by unloading the facet and possibly the disc, the load is transferred to the spinous processes. Little is known about the effect on bone integrity. Considering current indications for interspinous devices is stenosis, primarily seen in older patients, bone quality is a legitimate concern. Talwar et al11 found that bone mineral density was significantly related to spinous process failure load. They also found that the insertion load was well below the failure load of the spinous processes. However, in patients with very low bone density, the fracture threshold may be reached; the authors encouraged caution be used when implanting the devices in patients with low bone mineral density. As described by Whitesides,12 the earliest type of interspinous technology dates back to the 1950s when Dr. Fred Knowles inserted a steel plug that induced flexion to relieve symptoms while patients with stenosis improved with time. Some of the implants displaced and the device fell into disuse. The Wallis interspinous device (Abbott Spine, Austin, TX) was initially designed as a titanium device by Sénégas in the 1980s. It was secured into place by two Dacron bands around the superior and inferior adjacent spinous processes. The indications for the Wallis implant have been reported to include postdiscectomy with significant loss of disc material, repeat discectomy after recurrence, discectomy for herniation of a transitional disc with a sacralized L5, disc degeneration adjacent to a fused segment, and patients with low back pain with isolated Modic I changes.3 However, the implant has yet to be evaluated for all these conditions. In a nonrandomized study of patients with recurrent disc herniation, the original design of the implant combined with repeat discectomy was compared with discectomy alone.3 The values for the group receiving the implant with discectomy appeared to be more favorable than in the discectomy only group, no statistical comparison was provided. Sénégas et al reported that the device decreased analgesic requirements and low back pain in patients undergoing repeat surgery for recurrent L4-L5 disc herniation.13 The potential role of the Wallis implant in preventing recurrent disc herniation was recently evaluated in a group of 37 patients undergoing discectomy augmented with a Wallis device implantation for the treatment of large herniations and in whom at least 50% of disc height was retained.14 During follow-up (mean 16 months), the incidence of recurring leg pain was 13%, with two patients undergoing reoperation. The authors also had a rate of 13% recurrent symptoms following discectomy not supplemented with the Wallis implant. Therefore, they concluded that it did not provide any protective effect against recurrent disc herniation following discectomy. Sénégas et al15 reported a survival analysis of the first design of the Wallis implant in 142 patients with 14-year follow-up. Survival based on the failure endpoint of “any subsequent lumbar surgery” was 75.9% and for the end-point of “implant removal” was 81.3%. The survivability of multiple-level procedures did not differ from single-level procedures. This long-term follow-up provided favorable support for the ability of these devices to perform as intended for more than a decade. Fig. 23.1 Posterior (A) and side (B) views of an implanted Wallis device secured between the spinous processes. The Wallis device is now made of polyetheretherketone (PEEK) in an attempt to increase its elastic potential (Fig. 23.1). The new design of the Wallis device has been evaluated in biomechanical testing and computerized modeling.16 The implant resulted in reduced stresses in the disc at the implanted level and increased loads on the spinous processes. A prospective randomized trial is underway in the United States to evaluate the Wallis device in the treatment of mild to moderate symptomatic disc degeneration. The X-STOP (Medtronic Sofamor Danek, Memphis, TN) is a titanium interspinous device designed for the treatment of symptomatic lumbar stenosis, specifically neurogenic claudication. This device has an oval shape with two wings, one of which is attached to the body of the implant for fixation between the spinous processes (Fig. 23.2). Initial studies found that the X-STOP increased the spinal canal dimensions and decreased pressure in the posterior annulus at the implanted level.6 Changes in pressure were not seen at adjacent levels. In a cadaveric study the implant created 2 degrees of flexion resulting in a net decrease in motion at the implanted segment when moving from a position of lumbar flexion to extension.17 Adjacent levels were not affected.
Pathoanatomy of Biomechanical Load on the Intervertebral Disc
Biomechanics of Interspinous Decompression from Interspinous Implants
Types of Interspinous Implants Earliest Design
Wallis Interspinous Device
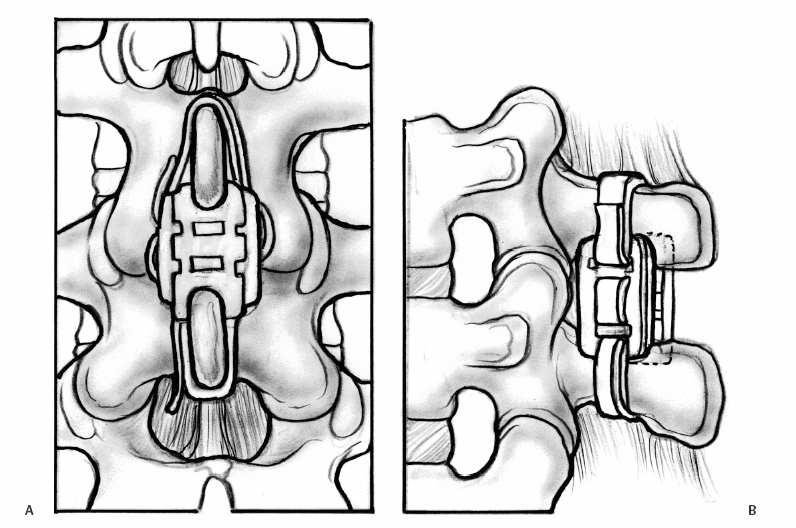
X-STOP
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








