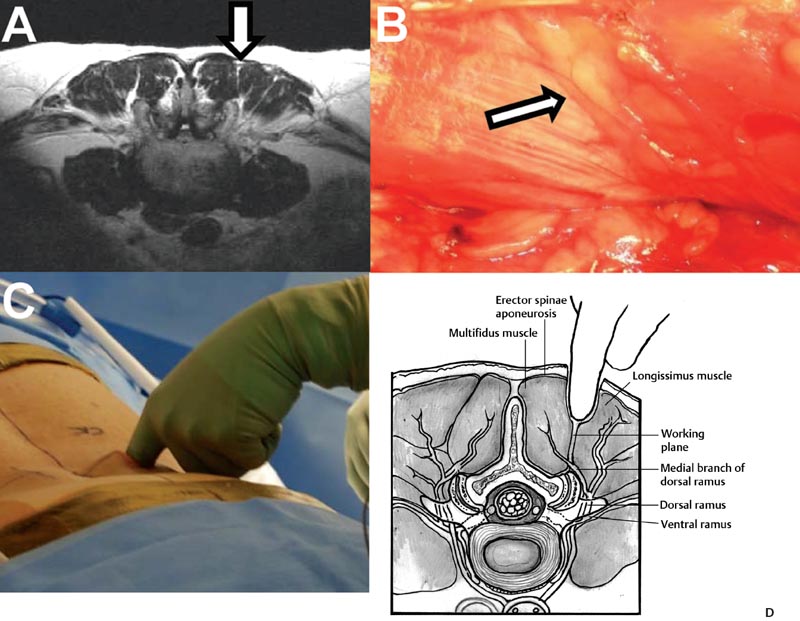
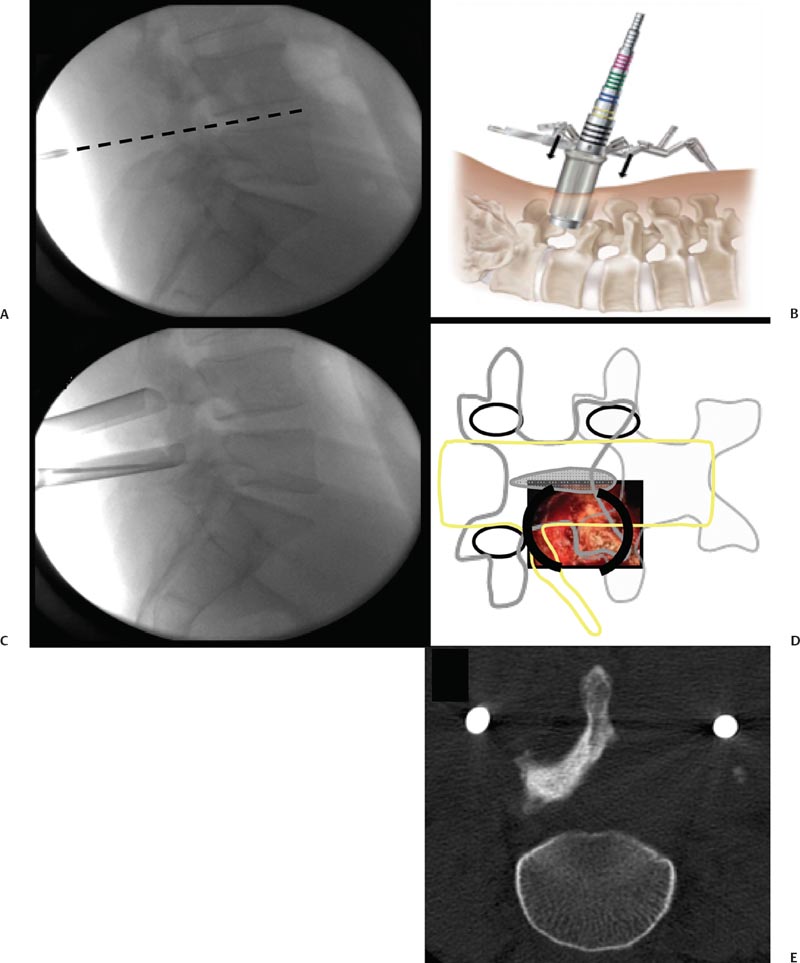
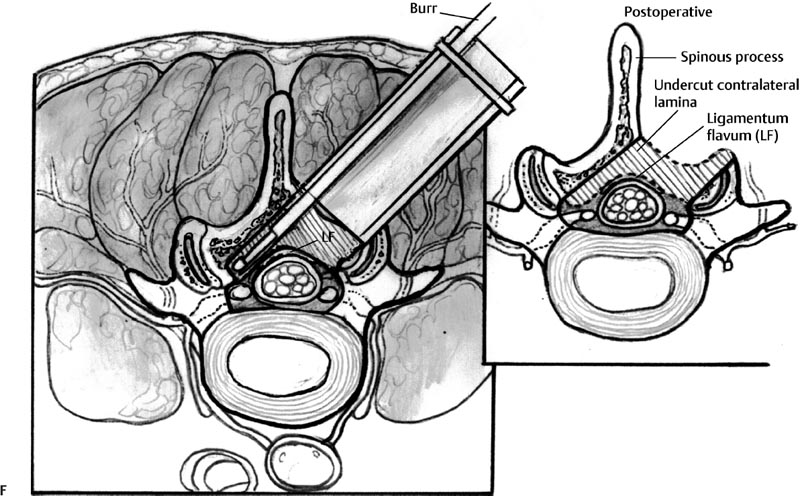
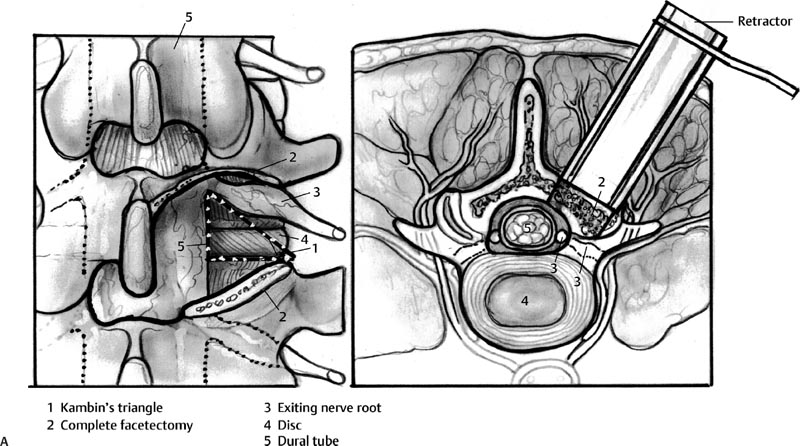
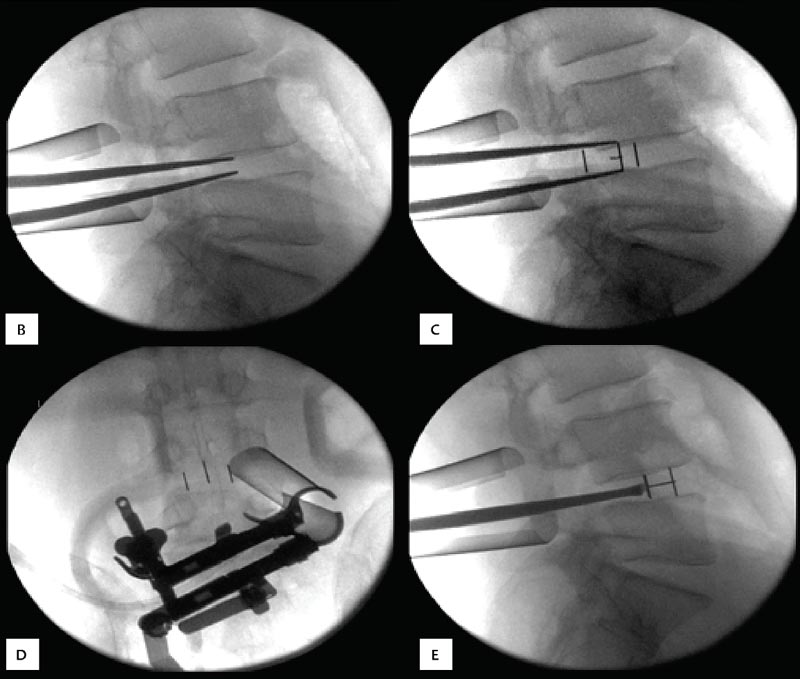
29 Minimally Invasive Spinal Fusion Procedures Mark P. Kuper, Mark C. Valente, William R. Taylor, and Choll W. Kim Conventional open spinal approaches require extensive dissections to allow for the identification of anatomic landmarks for instrumentation and graft placement. Multiple studies have demonstrated that these approaches lead to paraspinal muscle atrophy and scarring with impaired spinal function.1–6 Open surgery requires prolonged tissue retraction that is associated with elevated contact pressures, tissue ischemia, decreased muscle density, and paraspinal electromyographic abnormalities.1,5–8 Gejo and associates3 studied 80 patients who underwent open lumbar surgery. They noted that prolonged retraction time resulted in a decrease in trunk muscle strength. The study also correlated prolonged retraction times to an increased incidence of low back pain. In addition, Datta et al7 demonstrated that muscle retraction time was proportional to pain and disability scores. Minimally invasive surgery (MIS) techniques have the potential to decrease the amount of muscle injury by using tubular retractors that produce less tissue ischemia.9,10 In addition, these techniques eliminate muscle stripping that may denervate the paraspinal musculature. Several postoperative comparisons between open and MIS fusion techniques have supported this claim. Kim et al10 compared open and MIS posterior fusion patients. The study demonstrated that there is a significant decrease in the multifidus muscle cross-sectional area and a clinically significant decrease in trunk extension strength seen only in the open posterior fusion patients.10 In a similar study, Stevens et al9 compared the magnetic resonance images (MRIs) taken from patients that underwent posterolateral lumbar fusion by either a standard open or a minimally invasive approach. They reported “striking visual differences in muscle edema” in the open posterolateral lumbar fusion group of patients compared with the minimally invasive patient group.9 Hyun et al11 retrospectively assessed a group of patients that underwent unilateral transforaminal lumbar interbody fusion (TLIF) with ipsilateral instrumented posterior spinal fusion via a standard open midline approach. An additional contralateral instrumented posterior spinal fusion was performed at the same level, employing a paramedian intermuscular approach. Postoperatively, there was a significant decrease in the cross-sectional area of the multifidus on the side of the midline approach. On the contralateral side where the paramedian approach was used, no reduction in the multifidus cross-sectional area was measured. The aforementioned muscle and soft tissue injury that result from open surgery have led to an intense focus to perform less tissue-disruptive spinal fusions. MIS fusions strive to follow several key concepts. First, the exposure should be limited to only the target surgical site, limiting the unnecessary resection of bone, tendons, and muscle. Through preoperative planning, the most direct surgical approach to the pathology is identified. When possible, the surgeon should utilize intermuscular, internervous, and/or intervascular planes to develop the surgical corridor. Second, elevated contact pressures that result from retraction must be avoided. Finally, surgeons should exploit the interbody space to achieve fusions. The interbody space not only provides for the best fusion environment but also constitutes an isolated tissue compartment. The ultimate goal of any MIS spine fusion is to limit surgical morbidity while achieving radiographic arthrodesis rates comparable to conventional open surgery. Watkins12 first described the posterolateral fusion (intertransverse process fusion) in 1953, which yielded superior to previous fusion techniques. This soon became the standard fusion procedure of choice for decades to come. Despite its popularity, it required an extensive soft tissue dissection to span the distance between the transverse processes, especially when a midline incision is used. MIS posterolateral lumbar fusions, on the other hand, can be done via a Wiltse approach using blunt dissection and gentle retraction. This approach therefore has the potential to limit muscle injury from denervation and retraction-induced ischemia. In the posterior intermuscular (Wiltse) approach, the intraoperative C-arm is used to localize the midpoint between adjacent transverse processes to be fused ~4 cm off the midline. A 2.5-cm longitudinal skin incision is then centered over this point. The dissection and decortication of the transverse processes can be achieved through expandable tubular retractors (Fig. 29.1). Fig. 29.1 (A) The cross-sectional anatomy as viewed on the axial MRI scan shows the intermuscular plane between the multifidus and longissimus muscles (solid arrow). (B) Once the incision is made, blunt finger dissection develops the plane between the multifidus and the longissimus muscles thereby preserving the neurovascular supply to the musculature (arrow to nerve). Intraoperative photo (C) and illustration (D) of finger dissection technique. By exploiting the interbody compartment, surgeons gain a large surface area to increase fusion rates. Interbody fusions also aid in deformity correction and better restore intervertebral height, which, in turn, can increase neuroforaminal and central canal volume. When considering a posterior approach for interbody fusions, two approaches are common: the TLIF and the posterior lumbar interbody fusion (PLIF). The TLIF has gained popularity in past years due to several advantages over PLIF. Because the TLIF approach is more lateral, less dural retraction is required to perform a discectomy and interbody fusion. This theoretically decreases the risk of iatrogenic neurologic injury and allows this technique to be safely used above L3. The PLIF technique, on the other hand, requires bilateral neural retraction. The 10% incidence of transient neurologic complications historically associated with the PLIF is likely due to excessive retraction and manipulation of the roots and thecal sac.13 Multiple authors have recently reported on the safe and effective use of MIS TLIF techniques for refractory mechanical low-back and radicular pain associated with spondylolisthesis,degenerative disc disease, and recurrent disc herniation.14–17 Schwender et al17 reported on 49 patients who underwent MIS TLIF through a paramedian, muscle-sparing approach using an expandable tubular retractor system. Of these patients, 26 patients had degenerative disc disease with herniated nucleus pulposus, 22 had spondylolisthesis, and 1 had a Chance-type fracture as the primary diagnosis. The minimum follow-up was 18 months with a mean follow-up of 22.6 months. Operative time averaged 240 minutes (110 to 310 minutes), average estimated blood loss was 140 mL (50 to 450 mL). No patients required a blood transfusion and there were no intraoperative complications. Length of hospital stay was 1.9 days on average (1 to 4 days). All 45 patients who had preoperative radicular symptoms had resolution of their symptoms. All patients with mechanical low back pain (LBP) had postoperative improvement of their pain. There were four complications noted postoperatively (two from malpositioned screws, one from graft dislodgement causing new radiculopathy, and the last from radiculopathy caused by contralateral neuroforaminal stenosis). Visual Analog Scale pain scores improved from 7.2 to 2.1, and Oswestry Disability Index scores improved from 46% to 14% at last follow-up. The patient is placed prone. Intraoperative fluoroscopy is used to confirm the appropriate level and to position the patient in a true anteroposterior (AP) position relative to the floor. Longitudinal and transverse lines are drawn to delineate the landmarks to the spine. With the C-arm in the perfect lateral position, the initial dilator is positioned ~4 cm from the midline and directly in line with the disc space (Fig. 29.2A). A 2- to 3-cm longitudinal skin incision is centered over this entry point. The skin and dorsolumbar fascia are incised sharply with a scalpel followed by blunt digital dissection through the intermuscular plane between the multifidus and longissimus. This will place the tip of the finger at the lateral aspect of the superior articular process (SAP) as shown in Fig. 29.1. The facet joint is palpated and a periosteal Cobb elevator is used to gently release the tendinous attachments of the facet joint and hemilamina of the cephalad level. Using the lateral fluoroscopic image, dilators are inserted to sequentially surround the facet joint. With gentle downward pressure, firm twisting motions of the dilators will help dilate and clear soft tissue away from the facet joint (Fig. 29.2B). Once the final dilator is placed, the retractor is inserted over the final dilator and placed in line with the disc space (Fig. 29.2C). A rigid articulating arm is attached to the retractor blade and fixed to the operative table. The surgeon should always maintain constant downward pressure to prevent the retractor from backing out from the surgical site. A light source can then be attached to the retractor blades to illuminate the deep exposure. Final blade adjustments are then performed. Radiographically, the cephalad blade should expand to the inferior aspect of the superior pedicle. Likewise, the caudal retractor blade should expand for exposure to the superior aspect of the inferior pedicle. The surgical corridor will visualize the base of the spinous process, the cephalad inferior articular process and pars interarticularis, and the lateral edge of the caudal SAP (Fig. 29.2D,E). A bayoneted electrocautery tip is used to gently expose these bony landmarks. A curette is used to sweep soft tissue out and under the retractor blades and a pituitary rongeur is used to remove any remaining soft tissue within the confines of the retractor. A high speed matchstick drill, combined with fine curettes and Kerrison rongeurs, is used to remove the entire IAP and pars interarticularis. The ligamentum flavum is kept as a protective layer over the dural tube. Once a laminotomy window is identified, fine Kerrison rongeurs are used to complete the dorsal decompression. Removing bone at the base of the spinous process provides direct access to the opposite neuroforamen. A decompression of the contralateral nerve root is possible by undercutting the facet joint and thus achieving a full decompression of the canal (Fig. 29.2E,F). The facetectomy is taken from the inferior margin of the cephalad pedicle and the superior margin of the caudal pedicle. This “pedicle-to-pedicle” exposure allows for a true transforaminal trajectory when performing the discectomy and placing the interbody spacer. The anulus is exposed medial and inferior to the exiting nerve root in the Kambin triangle (Fig. 29.3A). Abundant epidural veins are present and should be preemptively coagulated with a long, bayoneted bipolar electrocautery. Extreme care should be taken to avoid using the bipolar electrocautery near the dorsal root ganglion of the exiting nerve root (Fig. 29.3A). The discectomy is subsequently performed using a variety of specialized angled instruments. Even through this limited exposure, an aggressive discectomy and endplate preparation can be accomplished. The disc space is sized with a smooth trial (such as a rounded paddle distracter) and the spacer is diagonal across the interspace (Fig. 29.3B-E). A preferred technique is to use banana-shaped spacers that can be maneuvered into the anterior midline position. This places the spacer on the strong apophyseal ring and furthest away from the posterior tension band as well as creating additional space for bone graft. Autologous bone graft (usually from the facetectomy is combined) and/or bone morphogenetic protein are typically used to achieve the fusion. Special consideration should be given to the insertion of the interbody spacer. In open TLIF techniques, the dorsolumbar fascia is incised. This allows the disc space to be adequately distracted using lamina spreaders between thelamina or spinous processes. In contrast to open TLIF techniques, the MIS TLIF techniques maintain the integrity of the dorsolumbar fascia. This makes distraction of the disc space extremely challenging. Without this distraction, the exiting and traversing nerve roots are at risk of injury during interbody spacer insertion. There are several techniques to overcome this obstacle. One reliable strategy is to use an insertion tool that acts as a shoe horn to open the disc space as the implant is inserted (Fig. 29.3B-E). Other techniques include the use of contralateral or ipsilateral pedicle screws (placed at the beginning of the procedure) to maintain distraction. In some cases, adequate lumbar kyphosis using a Wilson frame may be sufficient to allow safe interbody implant insertion. Percutaneous pedicle screw instrumentation is used for stabilization (discussed below). A posterior fusion can be performed through a matching contralateral incision through which the surgeon can decorticate the contralateral facet and/or transverse processes. In most cases, the interbody arthrodesis alone will be sufficient to achieve a fusion. Fig. 29.2 (A) Lateral fluoroscopic imaging is required to plan the precise approach to the disc space. The first dilator being pressed to the skin on a paramedian longitudinal line drawn 4 to 5 cm from midline. (B) Subsequent dilation and placement of the retractor will expose the surgical corridor. (C) Lateral radiograph showing margins of retractor. (D) Microscope view of surgical field with outline of relevant spinal anatomy. (E) Postoperative computed tomography scan showing bony decompression. (F) The retractor is angled medially to undercut the base of the spinous process, thus allowing a contralateral decompression. LF, ligamentum flavum.
Minimally Invasive Posterior Spinal Fusion
Posterolateral Fusion
Surgical Technique
Transforaminal Lumbar Interbody Fusion
Surgical Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








