Fig. 1
LCN2 expression was significantly increased in the white matter at 24 h after subarachnoid hemorrhage (SAH). (a) Representative bands of Western blotting of the expression of lipocalin-2 (LCN2). (b) Optical densitometry quantification for the expression of LCN2, normalized to β-actin. Values are expressed as mean ± SD; # p < 0.05 vs. sham group; ANOVA; n = 3
LCN2 Receptor, 24p3R Expression in White Matter after SAH
Next, we investigated the expression of the known LCN2 receptor 24p3R in the white matter after SAH using immunofluorescence double labeling. Diffuse expression of 24p3R was observed in the white matter at 24 h after SAH induction. LCN2 receptor 24p3R expressing cells were merged to GST-π (mature oligodendrocyte marker), GFAP (astrocyte marker), CD-31 (endothelial marker), and α-SMA (pericyte/smooth muscle marker) expressing cells.
LCN2 Deletion Ameliorates Albumin Leakage in White Matter after SAH
Mice that underwent endovascular perforation (SAH) but not a sham operation developed white matter T2-hyperintensity at 24 h, whereas LCN2−/− mice developed less of this T2-hyperintensity, as also shown in our previous study [4]. Immunohistochemistry revealed that SAH also induced albumin leakage (BBB disruption) along the white matter in WT mice, and the area of albumin leakage correlated with the T2-hyperintensity on MRI (Fig. 2a). The area of albumin leakage was significantly larger in SAH- than in sham-operated mice (0.65 ± 0.38 vs. 0.03 ± 0.02 mm2; p < 0.05; n = 4–5). This albumin leakage was much less evident in LCN2−/− mice (0.12 ± 0.06; p < 0.05 vs. WT + SAH; n = 5; Fig. 2b).
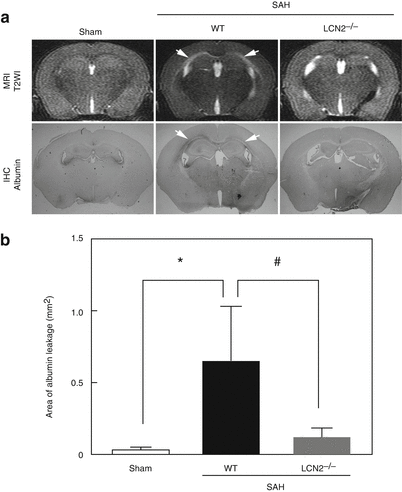

Fig. 2
(a) White matter injury detected by T2-weighted images (T2WI) and albumin immunohistochemistry (IHC) at 24 h after subarachnoid hemorrhage (SAH) in wild-type (WT) sham, WT with SAH, and lipocalin-2-knockout (LCN2−/−) with SAH mice. Arrows indicate white matter lesion. (b) Quantification of the areas of albumin leakage in each group. * p < 0.05 vs. WT sham, and # p < 0.05 vs. WT + SAH mice; Values are expressed as mean ± SD; ANOVA with Bonferroni; n = 4–5
Discussion
Accumulated evidence suggests that LCN2 is implicated in various neuronal injuries [3, 7, 11]. We recently reported that LCN2 plays an important role in SAH-induced acute white matter injury [4]. However, the detailed role of LCN2 in that injury remains uncertain. In this study, a significant increase of LCN2 expression in the white matter at 24 h after SAH was confirmed, and LCN2 receptor 24p3R was expressed in oligodendrocytes and BBB components. These results suggest that LCN2 might exert effects on these cells through its receptor. LCN2-24p3R-mediated apoptosis via the regulation of intracellular iron levels is one of the suggested mechanisms of LCN2-mediated cell death [2]. In this study, we demonstrated that LCN2 deletion attenuated acute BBB leakage after SAH. BBB disruption is recognized as a key mechanism of white matter injury in various central nervous system diseases that often occur early in the disease and contribute to promoting consequent white matter injury at a later time point [5, 12]. It is possible that acute BBB disruption is also involved in the pathology of SAH-induced white matter injury, and LCN2 might play an important role in initiation and progression of this process.
In summary, it was suggested that BBB leakage occurs in white matter after SAH, and it is one of the major mechanisms of LCN2-mediated white matter injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








