INTRODUCTION
Human history is replete with stories of the connection between stress and disease. A story about John Hunter (1728–1793), a surgeon and medical educator at St. George’s Hospital in London, is that he stated publicly, “My life is at the mercy of any rogue who chooses to provoke me,” and soon afterward died following a contentious meeting with hospital administrators.
A review of coroners’ records in Los Angeles in 1994 revealed a marked increase in deaths, including sudden death, related to atherosclerotic cardiovascular disease on the day of the 1994 Northridge earthquake. In 1999 during a 7.3 magnitude earthquake in Taiwan, patients on Holter monitors showed heart rate variability derangement due to withdrawal of parasympathetic activity and increase in sympathetic arousal. In the quarterfinal of the 1996 European football championships between the French and Dutch teams, a draw at the end of overtime resulted in a sudden death penalty shoot out, which was won by the French. An analysis of mortality in the total population of Dutch men and women aged 45 years or more revealed a relative risk of death from acute myocardial infarction (MI) or stroke of 1.51 among the men on the day of the match, compared with the 5 days on either side. There was no such effect on French men. This gave new meaning to the term “sudden death penalty.”
The psychological sequelae of exposure to life-threatening stressors are also problematic and disruptive to people’s lives. The terrorist attacks on the World Trade Center and the Pentagon on September 11, 2001, were witnessed directly by an estimated 100,000 people and vicariously by millions of Americans and others worldwide. In the period of time between October 16 and November 15, 2001, it is estimated that 7.5% of Manhattan residents south of 110th street were suffering from posttraumatic stress disorder (PTSD) and 9.7% were suffering from depression. In a representative sample of the American population surveyed 3–5 days following the attacks, 44% reported one or more substantial symptoms consistent with acute stress disorder (ASD), and 90% had one or more symptoms to some degree. Refugees and prisoners of war who have been exposed to torture are also likely to develop symptoms of PTSD, anxiety, and depression.
Less dramatically, research since the late 1960s has demonstrated correlations between significant changes in individuals’ lives and the subsequent onset of various types of physical and psychological illness. A consistent relationship has even been found between daily hassles and the onset of illness.
The interrelationship between mental stress and physical disease is complex and multifactorial. As a result, the study of stress and disease embraces a wide range of behavioral, emotional, cognitive, physiologic, hormonal, biochemical, cellular, environmental, and even spiritual interconnections, not easily understood or encapsulated in the controlled clinical trial.
It has been estimated that up to 70% of visits to primary care physicians are for problems related to stress and lifestyle. Most clinicians, however, have not been trained to extend their diagnostic workup and treatment interventions into the psychosocial context of these illnesses. Yet, adequate treatment and prevention require that providers regard their patients’ illnesses and suffering in the context of their life struggles. This perspective allows the clinician to intervene at multiple points along the continuum from mind to molecule. The biopsychosocial model of medicine, originally proposed by Engel, regards illness as multidetermined—by biochemical alterations on the molecular level and by psychological and social events on the molar level. This model encourages clinicians to move conceptually up and down the hierarchy from patients’ genetic susceptibilities and pathophysiologic processes to their unique life circumstances, stressors, and psychological meanings by which they construct their reality, to understand fully the origins of disease and the most appropriate therapies.
This chapter offers a framework for clinicians to think broadly and clinically about the stress–illness connection and suggests some approaches to assessing and treating stress-related illness. It offers a brief background of the research base for this perspective; provides a conceptual framework to guide diagnosis and treatment; suggests methods for communicating with patients about stress; and offers some options for stress assessment, prevention, and intervention.
 CASE ILLUSTRATION 1*
CASE ILLUSTRATION 1*
History: A 54-year-old female with a history of chronic migraine headaches, hypertension, hypercholesterolemia, mild obesity, and smoking presented to a new primary care physician. She was treated by her previous physician with Tylenol #3, 1 tablet twice a day, for the migraines, and she routinely called in halfway through the month for extra refills. Other medications included atenolol 25 mg once a day for blood pressure elevation and migraine prophylaxis, as well as over-the-counter antacids for occasional heartburn.
Patient Interview: The patient appeared angry and complained that the medication was not helping her pain. The new physician listened to her complaints and agreed at the end of the visit to refill her medications, but told her he would not prescribe more than the previous doctor. He asked her to return for her annual Pap smear, at which time he would take a history and do a physical examination.
*I am grateful to Kerry Kuehl, MD, for sharing the case presented in Case Illustration 1.
This case is a common occurrence in primary care medicine, and the patient’s chronic complaints pull for efforts by the new physician to ease her symptoms, especially with pain medication. Many physicians might regard this as a “difficult patient” (see Chapter 4). This physician astutely refrains from developing a treatment plan until after a complete history and physical, scheduled for the next visit. He provides a pain medication refill to meet the patient’s immediate needs and elicits her agreement to return for the subsequent evaluation. That next visit will offer an opportunity, in addition to the physical examination, to explore the psychological and social stress pathways that contribute to the patient’s symptom constellation. We will return to this case later, but first we will review the history and meaning of “stress,” its research background, and the rich array of assessment and intervention tools available for the physician to help this patient steer a new course to health.
DEFINITIONS
The concept of stress was borrowed by physiology and psychology from physics, where it generally refers to a force acting against some resistance. In materials science, stress is what is imposed on a material by the outer world; strain is the reaction of the material to the stress.
Hans Selye is generally credited with introducing the concept of stress into physiology. He defined stress loosely as “the rate of wear and tear in the body” and more rigorously as “the state manifested by a specific syndrome which consists of all the nonspecifically induced changes within a biologic system.” In this specific syndrome, termed by Selye the general adaptation syndrome (GAS), glucocorticoids are secreted by the adrenal cortex in response to adaptational demands placed on the organism by such disparate stressors as heat, cold, starvation, and other environmental insults—hence the expression “nonspecifically induced changes.”
A distinction is usually made between “stress” and “stressors.” Although stress is sometimes loosely used to refer to the environmental sources of threat to the organism, the term stressor is more appropriate in reference to these agents, whereas “stress” refers to the organism’s response.
More recent definitions of stress in humans emanate from a transactional model that takes into account the interactions between persons and their environment. In this view, stress occurs when a situational demand presents a call for action that the individual perceives as exceeding available resources. I have proposed the following working definition: “Stress is a process of interchange between an organism and its environment that involves self-generated or environmentally induced changes that, once they are perceived by the organism as exceeding available resources (internal or external), disrupt homeostatic processes in the organism–environment system.” This definition includes the traditional notion of stress as originating with an external demand (environmentally induced change) that exceeds the coping resources of the organism. It also includes, however, those expectations of events that arise from within, that are seen as essential to one’s life project, and that cannot be accommodated by the environment (exceeding external resources). The disruption of homeostasis in the organism–environment system can have its primary manifestation as a pathological end-state in the organism (illness or tissue damage) or as a destructive alteration of the environment (domestic violence as a stress response).
RESEARCH BACKGROUND
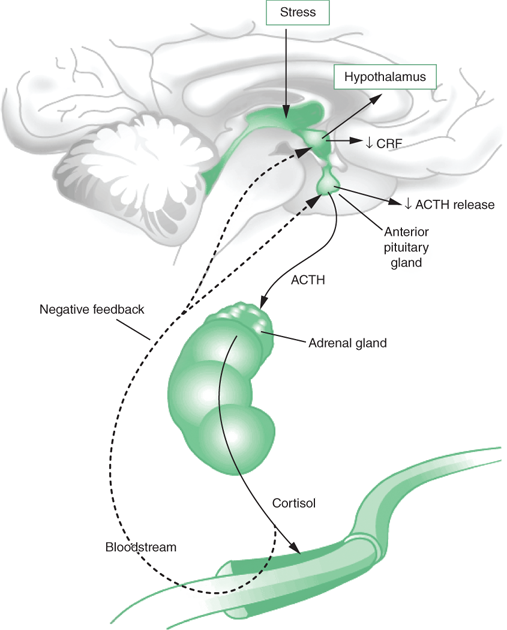
Psychoneuroimmunology (PNI) involves the study of the interactions of consciousness, the central nervous system (CNS), and the immune system (involving the body’s defense against infection and aberrant cell division). The compelling evidence of these studies is that the CNS influences immune function and that, conversely, the immune system can influence the CNS. The brain is normally part of the immunoregulatory network. Specifically, stimulation of the hypothalamic–pituitary–adrenal (HPA) axis leads to downregulation of immune system function in response to stress (see Figure 34-1). Stressful thoughts and emotions may reach the hypothalamus by axons projecting from the limbic system (primarily the amygdala) or from the forebrain. Corticotropin-releasing factor (CRF), produced in the hypothalamus under conditions of stress, acts on the anterior pituitary to release adrenocorticotropin hormone (ACTH), which in turn stimulates the production of corticosteroids in the adrenal cortex. Under conditions of acute stress, corticosteroids have immunosuppressive effects on the lymphoreticular system and marked antiallergic and anti-inflammatory effects.
In addition, CRF leads to release of catecholamines, which themselves may produce changes in lymphocyte, monocyte, and leukocyte functions. Opiates are also elevated with stress, and they are generally reported to be immunosuppressive. Finally, growth hormone and prolactin, which are immunoenhancing factors, are initially elevated at the onset of acute stress, but under conditions of prolonged stress their secretion is inhibited. Thus, the combined effect of elevated corticosteroids, catecholamines, and opiates, along with inhibition of growth hormone and prolactin, is to dysregulate the immune system.
Recent evidence indicates that chronic psychological stress can lead to increased production of proinflammatory cytokines, particularly interleukin-6 (IL-6), which is also triggered by infection and trauma. Proinflammatory cytokines have been implicated in a range of diseases in older adults that can be traced to inflammation, including cardiovascular disease, osteoporosis, arthritis, type 2 diabetes, certain lymphoproliferative diseases or cancers (including multiple myeloma, non-Hodgkin lymphoma, and chronic lymphocytic leukemia), depression, Alzheimer disease, and periodontal disease. IL-6 promotes the production of C-reactive protein, which is an important risk factor for MI. Depression and anxiety also enhance the production of proinflammatory cytokines, which is a possible mediator of the association of these disorders with increased morbidity and mortality (see Chapters 25 and 26). A recent study has shown that depressed subjects, compared with nondepressed controls, showed an impaired ability to regulate inflammation triggered by acute stress.
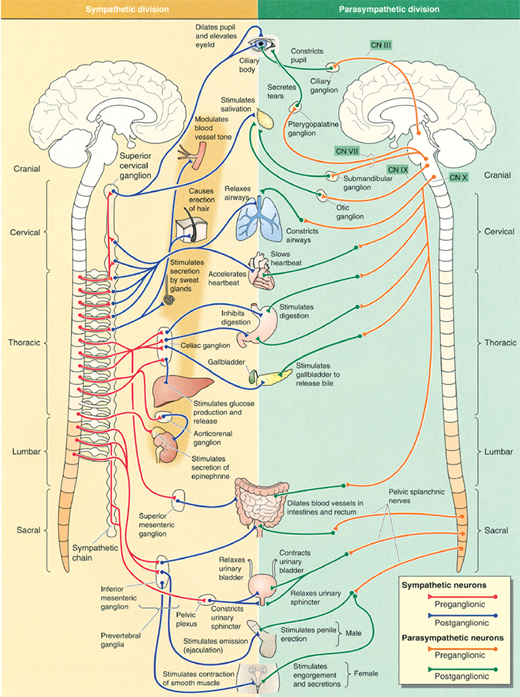
Another recent development in stress research involves the concept of “allostatic load,” which is the wear and tear on organisms that results from chronic overactivity or underactivity of allostatic systems. Allostasis refers to the body’s ability to produce hormones (e.g., cortisol) and other mediators (e.g., cytokines) that help it to adapt to novel situations or challenges. These systems, which include the autonomic nervous system (Figure 34-2), HPA axis (Figure 34-1), and the cardiovascular, metabolic, and immune systems, protect the body by responding to internal and external stress in an attempt to achieve stability through change.
Activation of allostatic systems in response to a stressor includes the release of catecholamines from nerves and the adrenal medulla, as well as the stimulation of cortisol release from the adrenal cortex via the HPA system described above. Four types of allostatic load can result from prolonged stress: (1) Repeated elevations of blood pressure over weeks or months accelerates atherosclerosis, increasing the risk of MI. (2) When adaptation to repeated stressors is lacking, there may be prolonged exposure to stress hormones. (3) There may be an inability to shut off allostatic responses after stress is terminated, leading to conditions such as hypertension or decreased bone mineral density. (4) Inadequate responses in some allostatic systems, such as cortisol secretion, may lead to compensatory increases in other systems, such as proinflammatory cytokines (which are downregulated by cortisol).
Inflammation is a common link for diseases high in mortality risk, including cardiovascular disease, cancer, and diabetes. Stress and depression, as well as dietary intake, can promote inflammation through proinflammatory cytokine production. Diet influences cytokine-induced inflammation by means of the balance between omega-3 (n-3) polyunsaturated fatty acids (PUFA) and omega-6 (n-6) PUFA, with n-6 (refined vegetable oils) promoting the production of proinflammatory cytokines and n-3 (fish, fish oil, walnuts, flax seed) mitigating their production. The link between depression and inflammation interacts with dietary balance, with higher n-6/n-3 ratios showing a relationship with depression. In epidemiologic studies, greater fish consumption has been associated with a lower prevalence of depression. A diet with higher n-6/n-3 ratio can also increase vulnerability to inflammatory responses to stress. Stress, in turn, promotes unhealthy food choices that promote inflammation, with higher stress associated with decreased fruit and vegetable consumption and increased consumption of sweets and fast food. Higher stress is also associated with higher peaks in postprandial lipidemia and delayed gastric clearing of these compounds, both associated with atherogenesis.
In a series of studies on asymptomatic men following notification of human immunodeficiency virus (HIV) seropositivity, a 10-week program of cognitive behavioral stress management and aerobic exercise training programs buffered distress responses and immune alterations. The same intervention had positive effects on mood and immune function in gay men whose disease had become symptomatic. Meta-analyses of the placebo effect have shown positive effects in various inflammatory and immune-related diseases, including asthma, cancer symptoms (pain and appetite), Crohn disease, chronic fatigue, duodenal ulcer, irritable bowel syndrome, and multiple sclerosis (relapse frequency). A meta-analytic review showed that three classes of interventions could reliably alter immune function. Hypnosis with immune suggestions showed a positive influence on total salivary immunoglobulin A (IgA) concentration and neutrophil adherence, along with a modest suppression of intermediate-type hypersensitivity erythema. These effects were mediated through relaxation. Some studies have shown differential delayed skin sensitivity reactions on the right and left arm of subjects depending on which arm was suggested under hypnosis to show no changes. Conditioning interventions, in which a neutral stimulus is initially paired with an immune-modulating stimulus and later elicits the immune changes on its own, were able to enhance natural killer (NK) cell cytotoxicity. Disclosure interventions, which encourage patients to write essays about previously inhibited stressful experiences, have shown some success in reducing antibody titers to Epstein–Barr virus and enhancing the body’s control over latent herpes simplex virus production.
In 1967, Holmes and Rahe published a scale of 43 life events, along with a method of quantifying life changes according to the amount of readjustment they require for the average person. This scale allowed greater quantitative precision in life change and illness studies and provided a pivotal methodological leap that broke through the circularity in which the stressful life changes had been measured in terms of illness outcome, rather than in terms of the inherent magnitude of the stressor. Questionnaires based on this and similar scales have gathered data on several populations globally. Retrospective studies have shown a relationship between recent life change and a host of pathological outcomes, such as sudden cardiac death, onset of MI, occurrence of fractures, pregnancy and birth complications, aggravation of chronic illness, tuberculosis, multiple sclerosis, diabetes, onset of leukemia in children, and onset of mental disorders such as depression and schizophrenia. Prospective studies, particularly those conducted on US Navy populations while deployed at sea, predicted future illness based on life change scores prior to deployment and subsequently verified the accuracy of those predictions by inspection of medical records.
Recent attention has focused on individual and situational variables that may mediate the relationship between life change and illness. Among the psychological variables that seem to mediate the stress response are locus of control (including the extent to which individuals prefer control in their lives and how much control they perceive they have over specific life events), need for stimulation, openness to change, stimulus screening, self-actualization, the use of denial, the presence of social supports, and emotional self-disclosure. In one study of Illinois Bell executives during the divestiture of AT&T, those executives who experienced high levels of stressors while remaining healthy differed from those with high stressors and high illness on a dimension of “hardiness.” This personal characteristic consists of “the 3 Cs”: a strong commitment to self, work, family, and other important values; a sense of control over one’s life; and the ability to see change as a challenge rather than a threat. More recently researchers suggest a “fourth C”: coherence, a belief that one’s internal and external environments are predictable and that things will work out as well as can be expected.
More recent studies of resilience (a concept related to hardiness) have focused on the neurochemical and hormonal feedback systems that dampen or switch off the stress response (HPA axis and sympathetic nervous system response described above). Among these resilience chemicals are DHEA (dehydroepiandrosterone), which lessens the effects of cortisol, and neuropeptide Y, which counters the effects of CRF at the anterior pituitary. These stress inhibitory mechanisms may be more prevalent in people who show a greater resiliency or hardiness in response to stressful events. Yet to be examined is the question of whether levels of these feedback chemicals are modifiable in response to stress management training or other psychological interventions.
Increased use of mobile phones, smartphones, and the Internet has established new norms for work and connectivity in industrialized countries. Although allowing broader and more rapid access to information than ever before in human history, as well as increasing flexibility for work location and hours among many workers, these technologies also exact a toll on human health. In a recent Swedish study of young adults, high mobile phone use was a predictor of stress, sleep disturbance, and depression at a 1-year follow-up. Increased use of social media to interact frequently with friends near and far presents a paradox. On the one hand these media facilitate engagement with social support networks, which are a well-established stress buffer. Chatting and instant messaging are among the high-frequency activities of those in Generation Y (born after 1980). On the other hand, these media also lead to communication overload, the taxing of working memory through multitasking, distractibility, and guilt induced by the social expectation of immediacy of response and constant availability.
The Stress in America survey published by the American Psychological Association (APA) revealed that those who serve as caregivers—providing care to both the aging and chronically ill—for their family members report higher levels of stress, poorer health, and a greater tendency to engage in unhealthy behaviors to alleviate that stress than the population at large. According to estimates from the National Alliance for Caregiving, 65.7 million Americans served as caregivers for an ill or disabled relative in the past year. In the APA survey, 55% of caregivers (median age 49 years) felt overwhelmed by the amount of care required by chronically ill or aging family members. Caregivers were more likely than those in the general population to report that they are doing a poor or fair job of managing stress and getting enough sleep. Mean stress level of caregivers (on a scale of 1–10) was 6.5 compared to 5.2 for the general public. Caregivers reported being poorer in health than the rest of the nation, with greater rates of high cholesterol, high blood pressure, overweight/obesity, and depression. Other survey findings were that caregivers were more likely than the general population to have chronic illness, to lie awake at night, to have poor nutrition, to skip a meal, and to get sick five or more times a year.
The demands of the workplace in industrialized societies are a persistent and intense stressor. Job strain is defined as a combination of high job demands and low perceived control. In a prospective study of healthy young adults (Cardiovascular Risk in Young Finns study), job strain was associated with increased carotid atherosclerosis among the men, but not the women.
In another prospective study, the degree of job stress over time increased the risk of the metabolic syndrome in a linear fashion. Subjects with lower grades of employment suffered disproportionately from the effects of stress as a risk factor for the metabolic syndrome, with men showing more susceptibility than women. Burnout is a syndrome associated with unrelenting stress and has been studied extensively as a phenomenon in a variety of work settings and professions, including physicians. It includes symptoms of emotional exhaustion, depersonalization, and a decreased sense of personal accomplishment. In a prospective study, burnout was associated with an increased risk of type 2 diabetes in apparently healthy individuals.
In Japan, the concept of karoshi, or death from overwork, has received government attention in recent decades. Rapid growth in industrialization following World War II led to production efficiencies placing pressure on Japanese workers, who often worked overtime without additional compensation. A new phenomenon of sudden death among high-level businessmen who were previously healthy brought attention to these stressful work environments. In 2008 in a well-publicized case a company was ordered to pay 200 million yen to a man who overworked until he fell into a coma. Among statistics published by the Japanese Ministry of Health, Labor, and Welfare in 2007 for the previous year, 189 workers had died from overwork, many from heart attacks and strokes, and 208 more had become severely ill.
Acute stress activates the sympathetic nervous system (Figure 34-2), which leads to increases in heart rate and blood pressure, coronary vasoconstriction, and decreased myocardial electrical stability. Several behavioral and emotional events have been implicated as probable triggers of acute coronary syndromes (MI and sudden cardiac death) in vulnerable individuals, especially events within a 1-to 2-hour period before the onset of symptoms.
Behavioral triggers include physical exertion (more common in men than women), sexual activity, sleep disturbance, and heavy consumption of alcohol. Well-studied emotional triggers include earthquakes, sporting events, war, high-pressure deadlines at work, and anger. In one study, the relative risk of acute MI in the 2 hours following an anger episode was 2.3, and in comparison with a control period 24 hours earlier it was 4.0. This effect was independent of age, sex, cardiovascular risk factors, and use of beta-blockers. The risk of anger triggering an MI was inversely related to socioeconomic status. In a large-scale study, the relative risk of anger triggering an MI was 9.0 compared to usual levels of anger, but when limiting the analysis to patients who had no premonitory symptoms the relative risk increased to 15.7.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree