Fig. 1
Thrombin activity following GMH, timed levels using standard assay
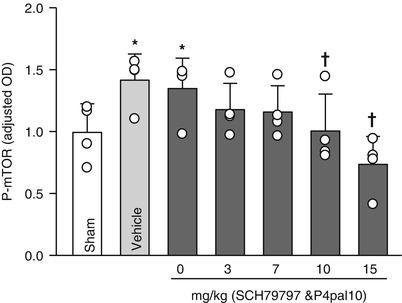
Fig. 2
mTOR activity post-GMH; dose-response following PAR-1 and PAR-4 co- administration; 72 h after collagenase infusion; (asterisk) <0.05 compared with sham; (cross) <0.05 compared with GMH (vehicle); SEM standard error of the mean; n = 4/group
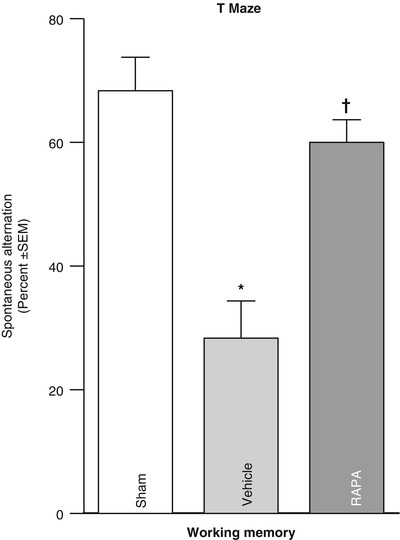
Fig. 3
T-maze (spontaneous alterations) measured 1 month after collagenase infusion; (asterisk) <0.05 compared with sham; SEM standard error of the mean; and the n = 4/group
Conclusion
Translational stroke studies, in particular those involving animal modeling, are greatly needed to safely integrate basic preclinical investigations ahead of eventual clinical applications [22–26]. This study therefore investigated the modulation of thrombin–PAR-1 and PAR-4 in reversing p-mTOR upregulation, as well as the effect of direct p-mTOR inhibition on neurological deficits. Prior studies hypothesized mechanisms of hydrocephalus involving increased production of infiltrating extracellular matrix (ECM) proteins of the cerebroventricular system, thus leading to the disruption of cerebrospinal fluid (CSF) outflow [1, 2, 14, 15, 27–32]. Our results suggested that thrombin-induced PAR-1,-4 stimulation could upregulate detrimental proliferative responses (i.e., p-mTOR mediated), possibly upstream of ECM dysregulation [1, 8, 11, 14, 15, 33–35]. To address the question of molecular mediators, we demonstrated that intraparenchymal infusion of collagenase generated thrombin with greatest activity in the acute phase between 6 and 24 h post-GMH, and then hypothesized that thrombin binds to PAR-1, -4 receptors, consequently upregulating p-mTOR. Because thrombin is most active in the early time frame, we examined levels at 72 h after GMH and found that p-mTOR was significantly greater in vehicle animals compared with shams. Then, inhibiting PAR-1, -4 through combined therapy using SCH-79797 (PAR-1 antagonist) and p4pal10 (PAR-4 antagonist) significantly ameliorated p-mTOR by day 3 after GMH. We then asked whether direct inhibition of p-mTOR could improve long-term outcome. Our findings showed that vehicle-treated animals significantly worsened outcome compared with shams, and treating with rapamycin (mTOR inhibitor) could significantly improve neurological ability. Thus, by decreasing early proliferative (i.e., p-mTOR) signaling, we improved long-term juvenile outcome. In sum, this study showed that thrombin-PAR-1, -4 signal inhibitions normalized early p-mTOR expression, and may improve long-term GMH outcome.
Acknowledgment
This study was partially supported by the National Institutes of Health grant RO1 NS078755 (Dr. Zhang).
Disclosures
None
References
1.
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67:1–8PubMedCentralCrossRefPubMed
2.
3.
Chen Q, Zhang J, Guo J, Tang J, Tao Y, Li L, Feng H, Chen Z (2014) Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res. doi:10.1007/s12975-014-0367-5
4.
Zhao J, Chen Z, Xi G, Keep RF, Hua Y (2014) Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 5:586–594PubMedCentralCrossRefPubMed
5.
6.
Uria-Avellanal C, Robertson NJ (2014) Na(+)/H(+) exchangers and intracellular pH in perinatal brain injury. Transl Stroke Res 5:79–98PubMedCentralCrossRefPubMed
7.
8.
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G (2014) Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 34:489–494PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








