(a): The percentage improvement from individual baseline in a memory composite score (the original figure and a description of how the composite score was calculated can be found in Miskowiak et al., 2014a). Mean and s.e.m are presented. Error bars denote standard errors of the mean. P-values indicate the results of the ANCOVA of the mean change from the individual baseline between the drug groups. The dotted line denotes the estimated RAVLT memory composite of healthy, age-matched individuals of average intelligence. (b): The percentage improvement from individual baseline in the cognition composite score of overall speed of complex cognitive processing score (the original figure and a description of how the composite score was calculated can be found in Miskowiak et al., 2014b). Error bars denote standard errors of the mean. P-values indicate the results of the ANCOVA of the mean change from the individual baseline between the drug groups. The dotted line denotes the estimated mean cognitive composite score of healthy, age-matched individuals calculated by z-transformation and summation of the average norms for healthy individuals (see procedure in Miskowiak et al., 2014b).
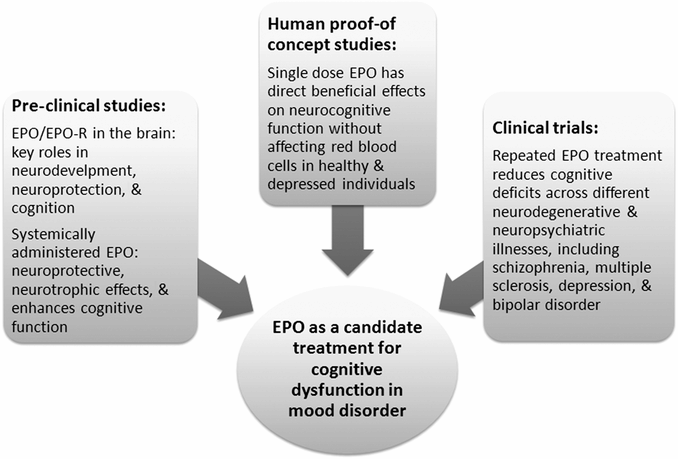
Schematic overview of the three lines of research that together point to erythropoietin (EPO) as a novel candidate compound to target persistent cognitive dysfunction in mood disorder.
Clinical limitations
Despite the promising effects of EPO on neurocognitive function, it is important to acknowledge three major limitations of EPO, which may limit its clinical use. The first problem is the increases in the red cell mass observed with repeated administration which lead to increased risk of hypertension and blood clotting. A potential solution could be to use non-hematopoietic EPO analogues such as CEPO, which exhibits a broad spectrum of neuroprotective activities in models of acute and chronic neural degeneration and enhances cognitive function in both brain-damaged and healthy animals (see review by Siren, Fasshauer, Bartels, & Ehrenreich, 2009). The second limitation is potential risk that EPO promotes malignant tumor growth, although the evidence for this adverse effect is still controversial (Jelkmann et al., 2008). Low-dose EPO is widely used in the treatment of chemotherapy-induced anemia to avoid red blood cell transfusions, but EPO is avoided in any non-anemic cancer patients (Jelkmann et al., 2008). A final clinical limitation is the high potential costs associated with EPO treatment; EPO itself is expensive. For example, a 1 ml vial of EPO (40,000 IU) costs approximately £420 in the United Kingdom and $420 in the United States. As cognitive enhancement is likely to require repeated EPO administration over several weeks, this would impose great costs on society and patients. Ongoing research effort therefore aims to delineate the neurocognitive effects of modified EPO molecules that can be manufactured and purchased at lower costs. In addition, EPO treatment requires careful monitoring by a physician for the entire treatment duration (including weekly medical examinations and blood tests), which is labor intensive and costly. Nevertheless, these clinical limitations may be outweighed by the potential socio-occupational benefits of EPO treatment for patients with a more chronic illness course who suffer from severe functional impairment.
Conclusion and future directions
Although the evidence is still scarce, the converging findings from the reviewed pre-clinical studies, human proof-of-concept studies, and clinical trials highlight EPO as a candidate add-on treatment to enhance cognitive function in mood disorder (for an overview of these lines of research see Figure 20.2). These studies demonstrated (1) that systemically administered EPO has neuroprotective and neurotrophic actions and improves cognitive function in animal models of acute neural injury, neurodegenerative, and neuropsychiatric conditions, (2) that weekly EPO administration over three to six months produces long-term cognitive improvement in schizophrenia and MS, (3) that the neurocognitive improvement originates from direct neurobiological actions as demonstrated in single dose human proof-of-concept studies, and (4) that weekly EPO administration over eight weeks produces mood-independent, long-lasting cognitive improvement in TRD and BD possibly via structural increase in the hippocampus.
The clinical application of EPO may be limited by its hematopoietic effects and financial costs. Important next steps for further clinical development of EPO are therefore to investigate (1) whether the unwanted hematopoietic activities of long-term EPO treatment can be avoided with infrequent EPO administration while maintaining the neuroprotective effects and (2) whether CEPO and other non-hematopoietic EPO analogues have the potential to reduce cognitive dysfunction in mood disorder. It is also an essential step for future trials to determine whether the EPO-associated cognitive improvement in mood disorder translates into increased socio-occupational function long term. If so, EPO treatment could have important implications for patients’ functional recovery, quality of life, and societal costs in the future.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





