Meningitis
Encephalitis
Encephalopathy
Myelitis
Myelopathy
Radiculitis
Radiculoneuritis
Cranial neuritis
Meningovasculitis
Myositis (not discussed in this chapter)
14.2.2 Epidemiology
Many of the viruses causing acute disease of the nervous system can be acquired worldwide, e.g. measles, mumps, coxsackievirus, echovirus, enterovirus, herpesviridae, HIV, lymphocytic choriomeningitis virus, papovavirus, etc. Some of the viruses, in particular those which are acquired by mosquito bite or tick bite, show a clear-cut regional or continental occurrence, e.g. tick-borne encephalitis virus, West Nile virus, Japanese encephalitis virus or other arboviruses. Other viruses which have been the aim of eradication campaigns occur only in well-circumscribed regions, e.g. poliomyelitis virus, enterovirus 68–71, Nipah virus or Zika virus, occurring in certain tropical areas both as epidemics and endemically. Besides the geographic distribution of these various viruses, the way of transmission may play an important epidemiological role (Handique and Handique 2011; Lyons and McArthur 2013). Due to various programmes of eradicating viral diseases by global vaccination campaigns as for measles, mumps and poliomyelitis or regional campaigns to prevent diseases like Japanese encephalitis or tick-borne encephalitis, a changing epidemiology requires the best possible and regular flow of information, i.e. an international surveillance system.
14.2.3 Pathogenesis and Pathophysiology
Tables 14.2a, 14.2b and 14.2c list the more important viral causes of acute meningitis, encephalitis, myelitis or any combination of these; in rare cases, meningovasculitis, encephalomyelitis or radiculomyelitis may be caused.
Enteroviridae |
Coxsackieviruses A and B |
Echoviruses |
In rare cases: poliomyelitis virus |
Arboviruses |
Tick-borne encephalitis virus |
West Nile virus |
Japanese encephalitis virus |
St. Louis encephalitis virus |
La Crosse virus |
Western equine encephalitis virus |
Colorado tick fever virus |
Dengue viruses |
Zika viruses |
Herpesviridae |
Herpes simplex virus type 2 (in particular relapsing meningitis – Mollaret meningitis) |
Epstein-Barr virus |
Varicella zoster virus |
Human herpes virus 6 (potentially also 7 and 8) |
Human immunodeficiency virus (after acute infection) |
Mumps virus |
Lymphocytic choriomeningitis virus |
Adenoviruses |
Arenaviruses |
Filoviridae |
Rubella |
Arboviruses |
Tick-borne encephalitis virus |
Powassan virus |
Colorado tick fever virus |
West Nile virus |
La Crosse virus |
St. Louis encephalitis virus |
Japanese encephalitis virus |
Yellow fever |
Dengue virus |
Equine encephalitis viruses |
Adenoviruses |
Herpesviridae |
Herpes simplex 1 |
Herpes simplex 2 (in neonates) |
Varicella zoster virus |
Epstein-Barr virus |
Cytomegalovirus (rare in immune competent) |
Enteroviruses |
Nipah virus |
Zika virus |
Measles virus |
Rubella virus (very rare) |
Filoviridae (very rare encephalitis, more frequent intracranial haemorrhage) |
Rabies viruses |
The route of infection differs greatly: enteroviridae being transmitted via the faeco-oral route, arboviruses – as the name arthropod-borne viruses indicates – are transmitted by mosquitoes or ticks and herpesviridae and most of the other viruses are transmitted via droplet infection, by direct contact and/or exchange of body fluids.
14.2.4 Clinical Features
14.2.4.1 Viral Meningitis
The term viral meningitis (lymphocytic/aseptic meningitis used as synonym) is a syndrome with the triad of fever, headache and stiff neck associated with photophobia and possibly signs and symptoms of the autonomic nervous system. It is associated with cerebrospinal fluid lymphocytic pleocytosis. A viral meningitis might evolve into a meningoencephalitis, myelitis, etc.; in these cases, the prognosis is determined by the encephalitic, radiculitic or myelitic part of the disease. A viral meningitis in its pure form has virtually zero mortality and an extremely low long-term morbidity.
14.2.4.2 Viral Encephalitis
The typical clinical presentation of encephalitis is an acute, sometimes subacute condition of fever, headache (frequently holocranial sometimes hemicranial), increasing behavioural abnormalities, mental disturbances, focal or generalised seizure activity, focal or generalised neurological deficits (aphasia, hemiparesis) and increasing qualitative and quantitative impairment of consciousness. It must be, however, noted that the clinical presentation also depends, at least to some extent, on the specific virus. Arboviruses, in particular Japanese encephalitis virus, West Nile virus and tick-borne encephalitis virus, may manifest with a predominating basal ganglia syndrome, tremor, bradykinesia and rigidity being the most important signs and symptoms. The course of the disease might evolve into a status epilepticus, a condition which carries a very high morbidity and acute mortality. In encephalitis, focal and/or generalised seizures may occur in up to 60 % of the cases (Handique and Handique 2011; Lyons and McArthur 2013; Misra et al. 2014; Nicolasora and Kaul 2008; Ross 2014; Rudolph et al. 2014).
14.2.4.3 Viral Myelitis
A direct invasion of viruses into the myelon is rather typical for enteroviruses (Huang and Shih 2014), frequently causing a well-circumscribed myelitis within the grey matter of the myelon, i.e. a poliomyelitic course of disease. Besides enteroviruses (poliomyelitis viruses, enterovirus 69–71), West Nile viruses and tick-borne encephalitis virus may also cause a poliomyelitic course of disease. Rare cases of “poliomyelitis” have been described in Chikungunya virus and in Japanese encephalitis. Completely different – from a direct viral invasion into the myelon – is the post- or parainfectious myelitis which frequently presents as a transverse myelitis (Cree 2014; Roman 2014; Tyler 2014).
14.2.5 Diagnostic Features
History of exposure (mumps, measles) and the clinical syndrome of the respective infectious disease (again mumps, measles or varicella, shingles, etc.) are – in case the patients develop signs and symptoms of acute meningitis – highly suggestive of the aetiology of meningitic disease. In pure meningitis, neuroimaging neither is indicated nor carries a chance of suggestive findings. However, if the viral meningitis progresses to meningoencephalitis or meningoencephalomyelitis or if the presenting features suggest encephalitis or myelitis, neuroimaging is essential, both in ascertaining and confirming the neurological syndrome and being helpful in establishing the appropriate diagnosis and prognosis. The most important is neuroimaging – if possible, at any rate, nuclear magnetic resonance imaging – in case of meningoencephalitis, since certain patterns of affection within the brain frequently allow the best possible “guess” in attributing the disease to a certain virus family (Table 14.3).
Flaviviruses: basal ganglia, thalami |
Enteroviruses: thalami |
Herpes simplex type 1: fronto-temporo-basal |
Rabies: brainstem, initially in particular medulla oblongata |
Electroencephalography is indicated in case of encephalitic signs and symptoms, with or without epileptic features. Both epilepsy-specific EEG changes and focal or diffuse abnormalities are clearly associated with a malfunction of the cortical areas, thereby allowing to objectivise the clinical syndrome of an encephalitis. In case of myelitis, somatosensory-evoked potentials and motor-evoked potentials help to classify an incomplete poliomyelitic or transverse myelitis course of disease, both allowing early diagnosis and accompanying ascertainment of the clinical course.
14.2.6 Non-CSF Laboratory Analyses
In viral CNS disease, the extra CSF laboratory values do not usually yield a specific result. It is essential to point towards the capacity of a wide range of viruses to involve – beside the meninges – also other organs, like the liver, kidney, etc. Therefore, minor laboratory signs of hepatic or renal involvement might easily underline the viral pathogenesis. However, only Epstein-Barr virus or cytomegalovirus clearly and regularly affect the kidney and/or liver so that the aetiological involvement of one of these viruses might correctly be surmised. Leucocyte count, C-reactive protein or procalcitonin do not show a clear pattern in the case of viral meningitis. In contrast to this, the differential count frequently shows a relative lymphocytosis; in case of Epstein-Barr-virus or cytomegalovirus infection, monocytes predominate differential white blood cell count.
14.2.7 Cerebrospinal Fluid (CSF)
In many cases of viral meningitis, in the earliest hours of the disease, a mixed or even predominantly polymorphonuclear leucocytosis in the CSF might be found. However, this is rapidly followed (within 12–24 h) by a predominance of the lymphocytes and monocytes. The CSF glucose and the CSF/serum glucose ratio are normal, and in the case of viral meningitis, CSF protein is only mildly elevated and CSF lactate normal. The microbiological diagnostic studies in a patient with viral meningitis or encephalitis are shown in Table 14.4.
Table 14.4
Diagnostic studies for microbiological/serological studies
Enteroviruses including poliomyelitis |
CSF viral culture |
CSF RT-PCR |
Throat and stool culture |
Arboviruses |
CSF IgM antibody-capture ELISA |
CSF PCR West Nile virus |
Paired acute and convalescent seraa |
Herpes simplex virus (HSV) |
CSF PCR HSV DNA |
Genital lesions |
Epstein-Barr virus |
Serology |
Heterophilic antibodies |
Antiviral capsid antigen (VCA) titres of 1:320 or higher |
EBV VCA IgM antibodies |
Absence of anti-EBNA IgG antibodies |
CSF PCR EBV DNA |
Human immunodeficiency virus (HIV) |
CSF PCR HIV-1 RNA |
Anti-HIV-1 IgG in CSF |
CSF viral culture |
Varicella zoster virus (VZV) |
CSF PCR VZV DNA |
CSF VZV IgG antibodies (serum/CSF ratio) |
CSF VZV IgM antibodies |
HHV-6 |
Isolation of virus in CSF culture |
Plus |
Paired acute and convalescent seraa |
Lymphocytic choriomeningitis virus |
Herpes simplex virus 2 |
Cultures are positive for herpes simplex virus 2 in the majority of cases of meningitis associated with primary genital herpes, but they rarely are positive in recurrent episodes |
Paired acute and convalescent sera mumps |
Paired acute and convalescent seraa |
14.2.8 Differential Diagnosis
Table 14.5 lists nonviral causes of lymphocytic/aseptic meningitis syndrome, a disease which might be mistaken for a viral CNS infection.
Table 14.5
Nonviral causes of acute lymphocytic/aseptic meningitis syndrome
Infectious causes |
Trypanosoma cruzi |
Cysticercus cellulosae |
Larva migrans |
Cryptococcus neoformans |
Coccidioides immitis |
Histoplasma capsulatum |
Sporothrix schenckii |
Mycobacterium tuberculosis |
Mycoplasma pneumoniae |
Rickettsia spp. |
Bartonella henselae |
Anaplasma spp. |
Treponema pallidum |
Borrelia burgdorferi |
Noninfectious causes |
Sarcoidosis |
Leptomeningeal carcinomatosis |
Lymphoma |
Wegener granulomatosis |
Behcet’s disease |
Systemic lupus erythematosus |
Drugs |
Nonsteroidal anti-inflammatory agents |
Sulphonamides |
Intravenous immunoglobulins |
OKT3 antibodies |
Isoniazid |
14.2.9 Therapeutic Management
14.2.9.1 Antiviral Therapies
If diagnosed early enough, enteroviral meningitis can be treated with pleconaril 200 mg t.i.d. for 1 week, herpes simplex virus type 2 meningitis with acyclovir 1,000 mg daily for 10 days and varicella zoster virus meningitis with acyclovir 2–3 g daily for 5 days, and a patient with acute HIV meningitis should receive a combination therapy (2 nucleoside analogue reverse transcriptase inhibitors or a non-nucleoside reverse transcriptase inhibitor combined with 2 nucleoside analogues). Every patient with signs and symptoms of viral encephalitis receives acyclovir intravenously (10 mg/kg b.w. t.i.d.). If the clinical course and both the neuroimaging/EEG and the CSF PCR clearly exclude HSV1 aetiology, acyclovir is stopped and either another specific treatment, if indicated, or at least symptomatic therapy is initiated.
Patients with viral meningitis do not need specific antiviral therapy; however, the best possible symptomatic care, e.g. analgesics, anti-emetics, etc., is essential. Clinical observation supplements the acute care. Specific antivirals or the administration of hyperimmunoglobulins is not recommended in a case with pure viral meningitis (De Souza and Madhusudana 2014; Nigrovic 2013; Putz et al. 2013; Ross 2014).
14.2.9.2 Symptomatic/Adjunctive Therapies
Every patient with a potentially life-threatening course of encephalitis and/or myelitis must be managed in an (neuro) ICU. If intracranial pressure is suspected, the placement of an ICP probe is indispensable; if ICP remains elevated or cerebral perfusion pressure is dangerously low (<50 mmHg), immediate follow-up neuroimaging is mandatory.
Encephalitic brain oedema may be diffuse or focal; however, anti-oedematous therapy with corticosteroids or osmotherapy is still a matter of discussion. No prospective randomised trials with respect to corticosteroids or osmotherapy exist for viral encephalitides. A small group of patients with severe viral encephalitis and hyperpyrexia may benefit from therapeutic hypothermia or, at least, therapeutic normothermia, i.e. targeted temperature management or decompressive craniectomy (Fig. 14.1).
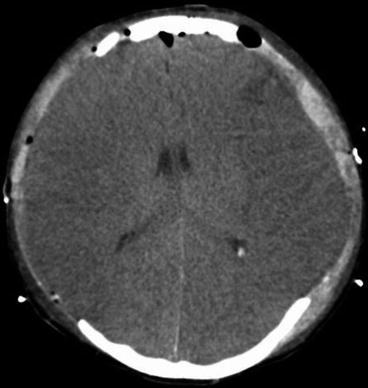

Fig. 14.1
Epstein-Barr virus encephalitis with life-threatening diffuse brain oedema, successful bilateral decompressive craniectomy
Isolated seizures, a common finding in encephalitis, need to be treated as any other symptomatic epileptic seizure. In the case of refractory status epilepticus, barbiturates may be considered; however, this group of drugs should only be given in encephalitic patients if an ICP monitoring probe is in place and the patient is on continuous EEG monitoring (Edberg et al. 2011).
14.2.10 Prognosis
Short-term and long-term prognosis in a patient with pure lymphocytic, viral meningitis is good, long-term mortality virtually zero and long-term morbidity also very low. In encephalitis and encephalomyelitis, long-term morbidity and mortality is definitely much higher than in pure meningitis. Without treatment, herpes simplex virus type 1 encephalitis carries a mortality rate of 70 %, the European tick-borne encephalitis or myelitis carries a mortality rate of up to 10 %, and the far eastern variant of TBE (Russian spring summer time encephalitis) has a mortality rate similar to Japanese encephalitis (30 %). Patients who survive an encephalitis or a myelitis have a likelihood of >10 % to suffer from severe neurological long-term sequelae, paraplegia or tetraplegia and epilepsy as well as focal or diffuse encephalopathies being the major long-term sequelae in patients with severe encephalitis and/or myelitis (Nigrovic 2013; Putz et al. 2013; Ross 2014; Zhang et al. 2014).
14.2.11 Prophylaxis
The avoidance of exposure to the various pathogenic agents (by avoiding areas of increased risk of transmission) and exposure to vectors in the case of arboviruses and the avoidance of close contacts in the case of droplet-, faeco-oral route of infection (mumps, measles, enteroviruses, etc.) are the most important steps of prevention. When available, active immunisation is a highly efficacious way to avoid the respective viral CNS disease (TBE, Japanese encephalitis, measles, mumps, poliomyelitis etc.).
14.3 Acute Bacterial Meningitis
14.3.1 Introduction
Acute bacterial meningitis is one of the most important acute inflammatory diseases of the central nervous system, early diagnosis and immediate initiation of the best possible empirical therapy being extremely important in reducing morbidity and the still high mortality. Despite improvements in antimicrobial chemotherapy over the past decades, neurological sequelae and mortality still remain unacceptably high for which mainly intracranial complications are responsible. The earliest possible recognition of such intracranial complications, e.g. diffuse brain oedema, status epilepticus, meningovasculitis leading to stroke, sinus or intracranial venous thrombosis, hydrocephalus, pyocephalus, is needed to allow – equally important – the earliest possible adequate adjunctive therapeutic measures. Only very recently, neurocritical care measures, by means of invasive intracranial pressure monitoring, have been shown to lead to an improvement of mortality in comatose patients with acute bacterial meningitis (from 30 to 10 %). Therefore, the earliest possible diagnosis, earliest possible specific and adjunctive therapeutic measures as well as monitoring and management in an intensive care unit setting are, besides prevention, the essential clue to further improve morbidity and mortality in acute bacterial meningitis.
14.3.2 Epidemiology
Worldwide, the incidence of acute bacterial meningitis is estimated to be 5–10 cases per 100,000 persons/year. These figures have changed dramatically throughout the past decade, in particular after the introduction of Haemophilus influenzae type B vaccine and the growing number of persons at risk who receive the appropriate polyvalent pneumococcal vaccine. Since the immunogenicity and safety of the multicomponent recombinant meningococcal serogroup B vaccine has been shown, the European Medicines Agency (EMA) issued an approval of this vaccine in January 2013. Serogroup B meningococci being responsible for more than 60 % of meningococcal diseases in central European countries, the other third mostly being caused by serogroup C meningococci (Bijlsma et al. 2014), it seems reasonable to assume that within the coming years, acute bacterial meningitis due to the “common pathogenic agents”, i.e. pneumococci and meningococci, will become rare events, the incidence dropping well below 1/100,000/year, as has been seen in the 1990s in Europe or a decade later in African countries for Haemophilus influenzae type B meningitis. Due to the demographic development and the fact that the ageing population will suffer more and more from various co-morbidities, enhancing the risk of either hitherto unusual or even unknown pathogenic agents leading to bacteraemia (e.g. Gram negatives, anaerobes, etc.), it might be assumed that in the future years, both community-acquired meningitis due to unusual bacterial pathogens and nosocomial bacterial meningitis in the case of invasive therapeutic or monitoring procedures (external ventricular drain, intracranial pressure probe, other monitoring probes, more invasive neurosurgical procedures, etc.) will replace the so far common and well-known pathogenic agents, in particular pneumococci and meningococci (Bhimraj 2012; Kasanmoentalib et al. 2013; Pomar et al. 2013). In the so-called meningitis belt – Sub-Saharan Africa, Arab Peninsula and northern part of India and Pakistan – meningococci still are the cause of epidemics, incidence rates being as high as 1,000/100,000/year in such an epidemic setting. However, even in sub-Saharan Africa, a change of epidemiology has been seen, these meningococcal epidemics moving from the immediate semiarid area of the Sahel zone towards the southern countries, extending towards Angola, Mozambique or Namibia.
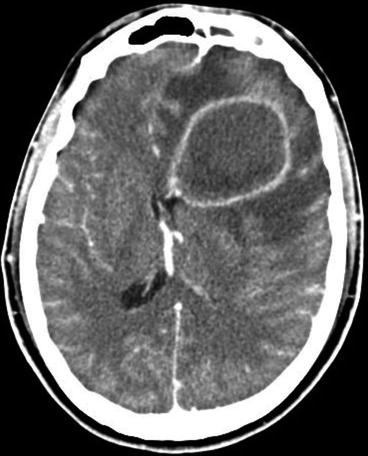
Specific attention has to be paid to the development of antibiotic resistance, which has been shown, in particular, to be the case for Streptococcus pneumoniae (pneumococci), becoming more and more prevalent in Asia, the USA and in certain European countries. Almost 20 years ago, already a third of cases of pneumococcal meningitides in the USA were caused by organisms not susceptible to penicillin, in European countries (Spain, France, Hungary, etc.) penicillin resistance rates even reaching more than 50 %. Very rare cases of penicillin resistance strains of Neisseria meningitidis (meningococci) have been reported so far. Besides this, age and seasonality and the place of acquiring the meningitis (community acquired versus nosocomial) are important epidemiological features in acute bacterial meningitis. Neonates and young children show a completely different pattern of pathogenic agents as do children and adolescents; these, again, show a different distribution of pathogenic agents compared to elderly and old patients. It is mainly age but also other predisposing factors like immunocompromised state, e.g. traumatic brain injury, preceding parameningeal infection (sinusitis, otitis, mastoiditis) which predispose patients to pneumococcal meningitis (Fig. 14.2). Neurosurgical interventions, open traumatic brain injury and invasive monitoring devices carry a high risk (up to 3 %/day) for nosocomial meningitis, caused by staphylococci or Gram negatives.

Fig. 14.2
Frontal sinusitis extending towards the meninges, causing pneumococcal meningitis with subdural empyema
Community-acquired meningitis is usually caused by pneumococci or meningococci; in neonates, however, group B streptococci, Listeria spp. and Gram negatives are most frequently seen. In the elderly, potentially immunocompromised patients, pneumococci, Listeria spp. and Gram negatives are the major causative agents for bacterial meningitis. In nosocomial meningitis (hospital-acquired meningitis), staphylococci or streptococci other than S. pneumoniae and, in particular, Gram-negative rods (e.g. Enterobacter spp., Klebsiella spp., Escherichia coli, Pseudomonas aeruginosa or Acinetobacter spp.) are the most frequently seen pathogenic agents.
14.3.3 Pathogenesis and Pathophysiology
Table 14.6a lists the most common pathogens of bacterial meningitis with respect to age and Table 14.6b with respect to predisposing conditions (the latter in adults) (Roos and van de Beek 2010; Sellner et al. 2010).
Table 14.6a
Most common pathogens in acute bacterial meningiti
Age | Most common bacteria |
|---|---|
<1 month | Gram-negative Enterobacteriaceae |
Streptococcus agalactiae (group B streptococci) | |
Listeria monocytogenes | |
1 year – 12 months | S. pneumoniae, N. meningitidis, H. influenzae type B, S. agalactiae, E. coli |
1 year – 18 years | N. meningitidis, H. influenza type B, S. pneumoniae |
19–50 years | S. pneumoniae, N. meningitidis |
>50 years | S. pneumoniae, Listeria monocytogenes, Enterobacteriaceae, N. meningitidis |
Table 14.6b
Acute bacterial meningitis: predisposing factor
Predisposing factor in adults | Typical pathogen |
|---|---|
Healthy, immunocompetent (community acquired) | S. pneumonia, N. meningitidis, L. monocytogenes |
Nosocomial (hospital acquired, post-neurosurgical, posttraumatic brain injury, device related (e.g. external ventricular drainage, etc.)) | Staphylococci, Enterobacteriaceae, Pseudomonas aeruginosa |
Shunt infection | Staphylococcus epidermidis, Staphylococcus aureus, Enterobacteriaceae, Pseudomonas aeruginosa |
Immunosuppressed patients | Listeria monocytogenes, Enterobacteriaceae, Pseudomonas aeruginosa, pneumococci |
Old/elderly patients | Listeria monocytogenes, pneumococci, Enterobacteriaceae |
Any bacterial pathogen which succeeds to cross the blood-brain barrier has the potential to cause acute bacterial meningitis. This sequence of events eventually leads to bacterial meningitis: colonisation of the host mucosal epithelium, successfully overcoming the local (mucosal) immune mechanisms (e.g. with the help of viral pharyngitis, laryngitis, rhinitis), invasion (and survival) within the intravascular space, arrival at the choroid plexus with successful crossing/penetration of the blood-brain barrier and, finally, survival and multiplication within the CSF. Replication and autolysis of bacteria lead to the release of bacterial cell wall components into the CSF, which is the most powerful stimulus to provoke the release of proinflammatory host factors (Mook-Kanamori et al. 2014; Sellner et al. 2010). Experimental and clinical studies have helped specify the complex pathogenic network in bacterial meningitis. Part of this network are cytokines (interleukin 1β, interleukin 6, tumour necrosis factor-α), chemokines, reactive oxygen species and reactive nitrogen intermediates (Mook-Kanamori et al. 2014; Sellner et al. 2010). Such chemotactic factors, and induced adhesion molecules, mediate the massive influx of leucocytes into the CSF (Sellner et al. 2010). It is this complex pathogenic network which contributes to CNS complications and brain damage, as there is hydrocephalus, meningovasculitis, venous/sinus thrombosis, brain oedema and eventually increased intracranial pressure. Besides these intracranial pathophysiological processes, in many cases, life-threatening systemic signs and symptoms can be attributed to related septicaemia, septic shock and even Waterhouse-Friderichsen syndrome. Bilateral adrenal haemorrhage, as typically seen in Waterhouse-Friderichsen syndrome, is thought to be rather a terminal phenomenon than the immediate cause of a potentially fatal adrenal insufficiency. Patients with meningococcal septicaemia, with overwhelming pneumococcal sepsis syndrome (in splenectomised patients) (Adriani et al. 2013), and patients with accompanying Gram-negative sepsis syndrome are highly likely to develop multiorgan failure, including shock, coagulopathy, kidney and liver failure, myocardial failure, pericarditis, arthritis, intestinal failure and metabolic derangement including SIADH, hyperglycaemia, etc. All these aspects contribute to morbidity and mortality.
14.3.4 Clinical Features
Typically, acute bacterial meningitis presents with headache, fever, photophobia, vomiting and malaise, neck stiffness and, eventually, qualitative and/or quantitative impairment of consciousness and seizures (Bhimraj 2012). In the very old and in the very young as well as the deeply comatose patient, neck stiffness may be very mild or even absent. Almost every patient (>95 %) with acute bacterial meningitis complains at least of two of the four symptoms: headache, fever, neck stiffness and qualitative/quantitative impairment of consciousness. The potentially life-threatening clinical signs and symptoms can evolve very rapidly within few hours, thus, rendering the disease a true neurological emergency. It is the earliest possible diagnosis with the earliest possible initiation of antimicrobial chemotherapy and the necessary initiation of adjunctive therapeutic measures that are the most important factors in reducing morbidity and mortality. Purpura fulminans (on presentation) is typical for meningococcal meningitis and sepsis syndrome but can also be seen in staphylococcal or pneumococcal disease. About 10 % of meningococcal infection shows a fulminant meningococcal septicaemia (Waterhouse-Friderichsen syndrome) which is characterised by septic shock, large petechial haemorrhages, multiorgan failure and disseminated intravascular coagulation (Fig. 14.3a–d). Such petechiae need to be differentiated from Osler’s spots (typically located on the fingers and toes) which are highly suggestive of infective endocarditis. In up to 15 % of patients with bacterial meningitis, focal neurological signs and symptoms may be found, suggesting brain abscess, subdural or epidural empyema, stroke or venous thrombosis (Alvis Miranda et al. 2013). Cranial nerve involvement is seen in approximately 10 % of patients with acute bacterial meningitis; seizures occur in up to a third of these patients.
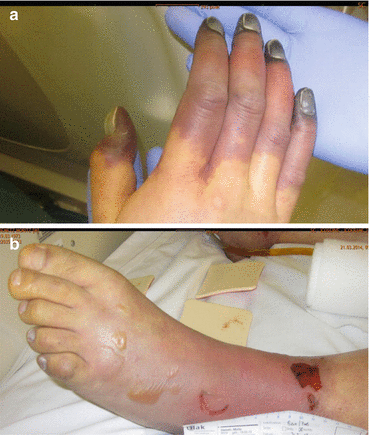
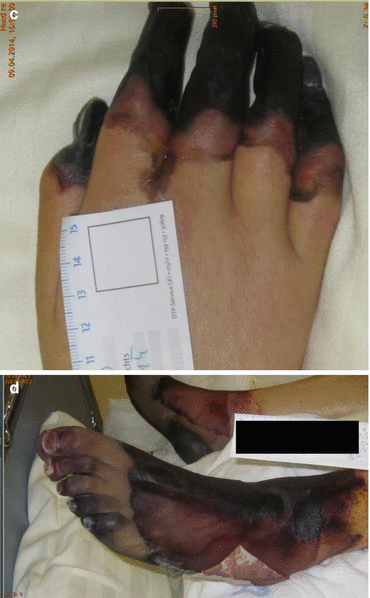


Fig. 14.3
(a–d) Meningococcal meningitis and meningococcal sepsis syndrome, purpura fulminans (a, b, day 3 after onset; c, d, day 19 after onset)
The course of meningococcal disease is frequently characterised by sepsis syndrome and septic shock, whereas the course of pneumococcal meningitis more often is characterised by intracranial complications.
Posttraumatic bacterial meningitis is often clinically indistinguishable from community-acquired meningitis. Therefore, in any traumatic brain injury patient, fever, deterioration of consciousness and impairment of vital function may indicate the advent or the presence of acute nosocomial meningitis. The presence of a CSF leak clearly supports the notion of a nosocomial meningitis which might, however, be hard to detect.
An infected permanent cerebrospinal fluid shunt usually causes a more insidious onset of disease with low-grade fever and with features typical for shunt malfunction, like headache, vomiting and impaired consciousness. Fever, usually a sign of CNS infection, is frequently absent in shunt infections. It should be noted that the peripheral part of the shunt may be infected without causing signs and symptoms of meningitis. Shunts draining into the venous system might produce a right-sided infective endocarditis; infection of shunts draining into the peritoneal cavity may produce focal or even diffuse peritonitis (Aftab and Shoaib 2013).
14.3.5 Diagnostic Features
The history, in particular the presence of predisposing factors or meningococcal disease in contact persons, the typical signs and symptoms of acute bacterial meningitis and/or sepsis syndrome are highly suggestive for the disease. The positive proof of the diagnosis can only be done by examining the cerebrospinal fluid. Every patient with suspected bacterial meningitis needs a spinal tap (Glimåker et al. 2013b); however, before that, neuroimaging is indicated if the patient shows impairment of consciousness and/or focal neurological signs and symptoms (Brouwer et al. 2014) (Fig. 14.4). In such a case, the administration of the first dose of the empirical antibiotic must never be delayed simply because of waiting for the neuroimaging. The very simple algorithm shown in Table 14.7 allows the best possible emergency care management of a patient with bacterial meningitis.