Figure 42.1. Differences in cerebral blood flow and glucose utilization rate according to anatomic region.
42.2.2 Determinants of Cerebral Blood Flow and Cerebral Autoregulation
The major determinants of CBF are:
- Cerebral perfusion pressure (CPP).
- Vessel diameter expressed as cerebral vascular resistance (CVR).
- Blood viscosity.
CFF = CPP/CVR
CPP is defined as the difference between mean arterial pressure (pressure at the beginning of the circuit [MAP]) and cerebral venous pressure (pressure at the end of the circuit [CVP]). However, from a practical point of view, the latter is very difficult to measure and is fairly accurately reflected by intracranial pressure (ICP), thus:
CPP = MAP – ICP
Cerebral vascular resistance is determined by the ability of the cerebral blood vessels to contract or expand in different situations or in response to stimuli. This property of the cerebral vasculature, which allows it to maintain CBF constant despite variation in CPP, is called cerebral autoregulation (Figure 42.2).
Normally, CBF is maintained relatively constant between 50 and 150 mmHg of mean arterial pressure. Outside these limits, CBF passively follows arterial pressure, which means that the vasculature will collapse at MAP <50 mmHg, with a consequent decrease in CBF. At the other extreme, MAP >150 mmHg overloads vasoconstrictive capacity, leading to vasodilation with a consequent increase in CBF.
Cerebral oxygen consumption is another determinant of CBF. As metabolic demands rise, CBF increases directly and proportionally to meet them.

Figure 42.2. Cerebral autoregulation.
42.3 The Ischemic Penumbra Area
42.3.1 Definition
After an acute ischemic attack, at least two areas in the brain parenchyma can be observed: a central one, the “core”, with established and irreparable structural damage, and therefore necrotic, and a larger peripheral around the core with the potential to recover lost functions, known as the “penumbra”. The penumbra is a region of brain tissue with functional damage at risk of being affected irreversibly but still potentially viable, so that the damage may be reversed with appropriate therapeutic measures.
42.3.2 Concentric Four-compartment Ischemia Model
Hypoperfused brain parenchyma, depending on the magnitude and duration of ischemia, can be compartmentalized into (Figure 42.3): a center or core (4) which represents the irreversibly damaged or infarcted tissue. Surrounding the core with variable extension it is the penumbra area (3) at serious risk of definitive stroke. Between the unaffected area (1) and the ischemic penumbra there is a zone of “benign oligemia” (2) which usually survives the attack, even without early reperfusion, depending primarily on the “time” factor.
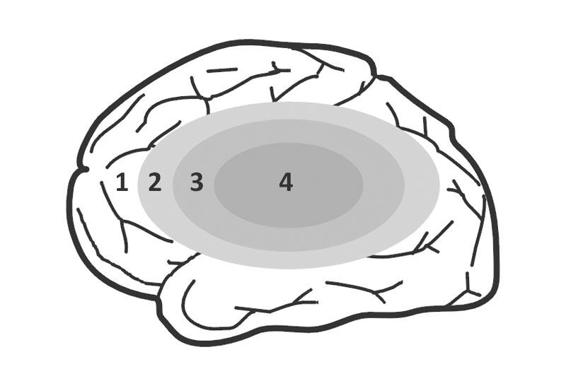
Figure 42.3. Four-compartment brain ischemia model.
1 = unaffected area; 2 = benign oligemia; 3 = penumbra area; 4 = ischemic core.
42.3.3 Relationship Between Cerebral Blood Flow and Penumbra Area
By definition, the penumbra is an ischemic region with decreased CBF. Experimental and clinical studies have demonstrated two critical thresholds during focal cerebral ischemia. When CBF drops below 20 ml/100 g/min, the neurons lose their spontaneous or induced ability to conduct electrical impulses: this is the “threshold of electrical failure” characterized by alterations on the electroencephalogram (EEG) and in evoked potentials. Typical EEG findings in this phase are the slow pathologic activity (theta and delta waves), with the concomitant disappearance of physiological alpha and beta rhythms.
When CBF reaches 10 ml/100 g/min, the cells are unable to maintain transmembrane ion gradients: this is the “threshold of ionic failure”, which produces ionic collapse, causing a massive influx of calcium into the cell, triggering cascades of different nature and complexity that interact with a single purpose – neuronal death.
Hence, it is very important to keep in mind that the value of CBF determining the “point of no return” depends on the time elapsed since the beginning of ischemic insult.
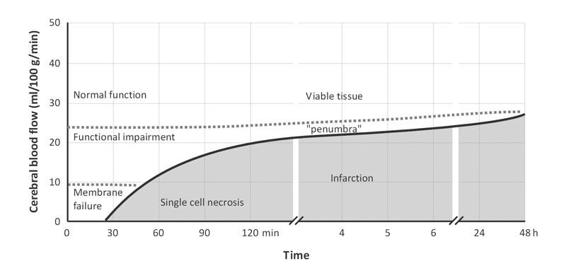
Figure 42.4. Schematic relationship between CBF and ischemia/infarction. CBF below the threshold and with the “time” since the event causes cell death and infarction.
Although the penumbra develops consequent to an ischemic event, positron emission tomography (PET) and magnetic resonance imaging (MRI) studies have shown that during the acute phase the CBF may be decreased, normal or increased, depending on the immediate reperfusion of the ischemic but not the necrotic tissue. And although reperfusion occurs relatively quickly, it is not always beneficial nor does it guarantee functional recovery of the affected area.
From a metabolic point of view, the penumbra is characterized by increased cerebral oxygen extraction and a decreased rate of oxygen utilization. In this way, areas with a metabolic rate <1.7 ml/100 g/min almost invariably progress towards infarction, whereas those areas which maintain an oxygen utilization rate >2.5 ml/100 g/min remain unchanged. Intermediate values are associated with an indistinct evolution towards necrosis or full recovery.
42.3.4 The Concept of Therapeutic Window
“Time is brain” is the American sentence created to raise public awareness, and to awaken health providers involved in the chain of care of these patients, to the fact that every hour of ischemia and tissue injury that elapses increases the degree and the extent of irreversible neuronal damage. An average of 6 hours is considered as the period during which threatened or at risk cells can be kept viable by restoring blood flow or initiating therapeutic measures to prevent the spread of the penumbra towards infarction. This period is called “therapeutic window”.
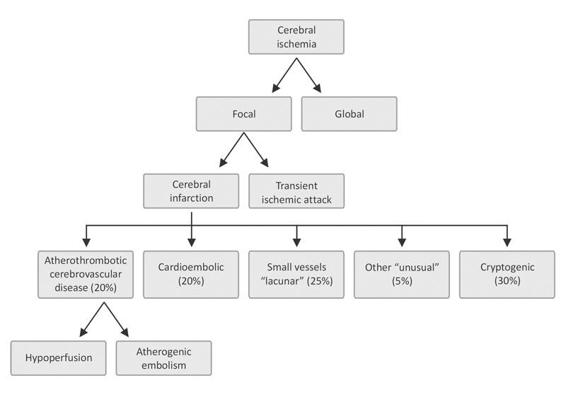
Figure 42.5. Classification of ischemic stroke according to the triggering mechanism.
42.3.5 Classification and Diagnostic Criteria of Ischemic Stroke
Grouped under the term “cerebral ischemia” are those entities that share qualitative or quantitative abnormalities in blood supply to the brain. Two main groups are distinguished: a) focal when ischemia affects only one region of the brain parenchyma, and b) global when ischemia affects the whole brain. Focal cerebral ischemia is further subdivided into: a) transient ischemic attack (TIA); and b) stroke.
42.4 Transient Ischemic Attack (TIA)
In light of current knowledge, primarily supported by advances in neuroimaging techniques, TIA is defined as a “brief episode of neurologic dysfunction caused by cerebral or retinal ischemia with signs and/or symptoms lasting no more than 60 minutes and without evidence of infarction on neuroimaging.” Recent guidelines suggest eliminating the time factor from the definition. There is a trend towards considering TIA as a benign entity: nothing could be more wrong. It is a serious entity which carries the risk of death or severe disability, and it should never be underestimated. Nonetheless, TIA provides a unique opportunity to start early treatment and prevent progression to stroke. It is estimated that after a first episode of TIA, 10 to 20% of patients will have an infarction in the next 90 days (half in the subsequent 24-48 hours), so that: the development of signs and/or symptoms of cerebral ischemia is a medical emergency.
The causes of TIA are the same as those for stroke (atrial fibrillation, carotid disease, etc.). They will be discussed in detail below, along with measures to prevent future attacks. The diagnosis of TIA, because problematic, requires high index of suspicion and common sense. The clinical history is important; but as it is sometimes impossible to collect data from a patient with altered speech or consciousness during the event, the neurologic examination may identify or clarify the existing deficits.
The clinical features of a TIA depend on the origin and the vascular territory affected. Often, carotid TIAs present with unilateral ocular symptoms such as transient monocular blindness or “amaurosis fugax”or sensory-motor deficits contralateral to the lesion, whereas vertebrobasilar TIAs are generally less localized and last shorter. Isolated unilateral sensory or motor deficits affecting at least two body regions (face, arm or leg) can guide us towards the diagnosis of lacunar TIA.
Since metabolic disorders (e.g., hypoglycemia, hyponatremia, severe hypoxemia, etc.) can obscure the clinical features, laboratory measurements should include: complete blood count (CBC), glycemia, uremia, creatinine, coagulation studies, C-reactive protein and blood gases according to the situation. In addition, routine studies should include chest radiograph, electrocardiogram (ECG), Holter monitoring if occasionally necessary to detect paroxysmal arrhythmias and an echocardiogram is mandatory to determine not only cardiac function but also the possibility of cardioembolic sources detection. In this context, and where feasible, a transesophageal echocardiography is preferable, as because it is more sensitive and specific, especially when checking the aortic arch, the mitral valve or the presence of a patent foramen ovale. It is essential to obtain computed tomography (CT) or MRI studies for diagnosis, as they will rule out or confirm other causes of neurologic dysfunction such as: brain tumor, subdural hematoma, bleeding, or others. Also important is examination and study of the carotid arteries with either Doppler ultrasound or MR angiography, which have a sensitivity of 83 and 86%, respectively, to detect stenosis >70%. Digital angiography is used only to confirm the findings and for selecting the surgical technique and tactics to employ.
42.4.1 Risk Assessment
TIA must be properly evaluated not only to define the most appropriate treatment but also to refer the patient to the most appropriate facility and to prevent recurrence and/or occurrence of stroke or cerebral infarction. Rating scales such as the California or ABCD score have been developed and independently validated. A recently modified version of these scores, called ABCD2, has shown better predictive power of stroke within 7 days of the event. The scale is composed of clinical variables (Table 42.1): the risk is considered high when the score is >4 points.
Variable | Score | |
Age >60 years | 1 | |
Arterial pressure >140/90 mmHg | 1 | |
Symptoms: speech disturbance | Without focal weakness | 1 |
With focal weakness | 2 | |
Duration of symptoms | 10 to 59 minutes | 1 |
>60 minutes | 2 | |
Diabetes | 1 | |
Table 42.1. ABCD2 score.
These scores do not incorporate the findings of neuroimaging which by themselves have a high predictive value. For instance, the presence of new infarction increases from 2 to 15 times the possibility of a short-term stroke, whereas blood vessel occlusion demonstrated by MR angiography raises that possibility fourfold.
42.4.2 Treatment
Antiplatelet Drugs
Aspirin reduces the relative risk (average 22%) of cerebrovascular accidents and long-term cardiovascular events after a first episode of stroke or TIA. Aspirin also prevents recurrence and improves functional outcomes. The optimal dose has not been determined; however, while the beneficial effect of the drug is indifferent for doses from 75 to 1300 mg a day, low doses between 200 and 325 mg are preferred due to the lower probability of side effects.
In patients allergic to aspirin or in whom TIA recurs under treatment with the drug, some authors suggest the use of clopidogrel 75 mg daily. A recent study suggested that combining clopidogrel with aspirin or vice versa doesn’t enhance the benefit in relation to aspirin or clopidogrel alone in reducing the rate of cerebral and myocardial infarction or cardiovascular death. The ESPS II European study showed that the association of dipyridamole and aspirin slightly improves aspirin’s effectiveness.
Anticoagulants
While the use of anticoagulants has not been tested in TIA, the wide experience gained in ischemic stroke suggests that in patients with atrial fibrillation, anticoagulation therapy reduces the risk of recurrence. In patients without atrial fibrillation, the use of anticoagulants afford no greater benefit than with aspirin. A meta-analysis of 21 placebo-controlled trials of several anticoagulants in patients with cerebrovascular ischemic stroke failed to show benefits.
Statins
Recent studies support the use of statins based on their pharmacological effects independent of inhibition of HMG-CoA reductase and thus their effects on cholesterol or atheromatous plaque. They can reduce endothelin levels, prevent the degradation of oxidized LDL by nitric oxide synthase, with an indirect vasodilator effect; they can inhibit cell proliferation and have other properties such as anti-inflammatory action, inhibition of thrombogenesis, and platelet aggregation and atherosclerotic plaque stabilization.
Several recent, properly designed large-scale studies with long follow-ups have demonstrated that statin use (specifically pravastatin, lovastatin and simvastatin) significantly reduces morbidity and mortality due to vascular events, including strokes and TIAs.
Carotid Endarterectomy
This topic will be discussed in more detail in another chapter. TIA secondary to carotid stenosis >70% clearly benefits from this therapeutic modality.
Control and Prevention of Risk Factors
All measures to reduce or eliminate the risk factors for brain or cardiovascular diseases are also effective in preventing the recurrence of TIA or its evolution towards stroke. Statins reduce the relative risk by 29%, even in the absence of dyslipidemia, while antihypertensive drugs lower the relative risk by 25-30%.
In diabetic patients the strict control of glycemia is a priority. Weight control, exercise, smoking cessation and moderate alcohol consumption are other voluntary preventive measures that should not be overlooked.
42.5 Atherosclerotic Carotid Disease
Carotid stenosis is an entity that clearly predisposes to TIA or stroke. An estimated 20% of ischemic cerebrovascular accidents are caused by atherosclerotic plaques in the initial portion of the internal carotid artery. Involved in its pathogenesis are many factors: mechanical forces that damage the endothelium, triggering reflexes and the release of inflammatory mediators, expression of adhesion molecules, and secretion of growth and chemotactic factors that induce the proliferation of smooth muscle cells and macrophages in the vessel wall. Other mechanisms included are: synthesis of a connective tissue matrix incorporating cholesterol, platelets, fibrin and lipoproteins, thus forming atherosclerotic plaques.
The clinical picture and Its signs and symptoms are similar to those described for TIA.
Assessment must be completed with high-resolution Doppler ultrasound, MR angiography, transcranial Doppler with the study of cerebral vasoreactivity and detection of emboli.
42.5.1 Therapeutic Approach
The therapeutic approach to patients with carotid atherosclerosis should be directed towards achieving the following goals:
- Stabilize and halt the progression of atherosclerotic plaque by modifying or removing the above risk factors, together with the use of statins, angiotensin-converting enzyme inhibitors and aspirin.
- Eliminate or reduce the stenosis by carotid endarterectomy or angioplasty with stent implantation.
Endarterectomy in Symptomatic Carotid Stenosis
The use of this technique for symptomatic stenosis ≥70% is strongly supported by three studies: NASCET, the American Veterans cooperative study, and the European ECST.
The NASCET showed that, after 2 years, the rate of ischemic stroke ipsilateral to the affected carotid territory was 26% in the medically treated group versus 9% in the surgical group: this amounts to a relative risk reduction of 65% and an absolute reduction of 17%. For patients with stenosis <50%, the three trials unanimously agreed on the lack of efficacy of surgery.
In moderate stenosis (50-69%), surgery does not provide a significant benefit according to the ECST. For this same group, the NASCET showed that the rate of fatal and nonfatal ischemic accidents was 22.2% in the group under medical treatment versus 15.7% in individuals undergoing endarterectomy, with an absolute risk reduction of 6.5%.
Factors associated with better outcomes were: gender (being male), age >75 years, more severe stenosis, stroke with hemispheric symptoms in the last 3 months, intracranial stenosis, the absence of microvascular ischemia (leukoaraiosis or small hypodense images in the periventricular white matter), and presence of collateral vessels.
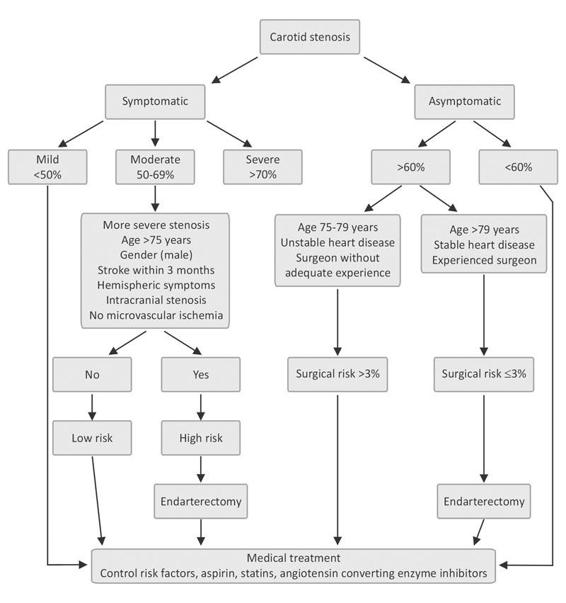
Figure 42.6. Systematic management of patients with carotid stenosis.
An additional aspect to consider in global assessment is the presence of ulcerated or complicated plaques, a finding that often aids in the selection of intervention.
When outlining the strategy to follow, the presence of co-morbidities and surgeon’s experience should not be omitted.
Role of Endarterectomy in Asymptomatic Carotid Stenosis
Patients with significant carotid lesions (>60%) and no symptoms were evaluated in a multicentre US-Canadian study (ACAS) involving 1661 subjects followed for a 5-year period. The rate of cerebral ischemic event and/or death was 5.1% among the operated patients and 11% for the medical treatment group (absolute risk reduction of 6%).
The key to success in this group of patients resided in the excellent results obtained by the surgical teams involved. Surgery-related morbidity and mortality was about 1.4%. Based on these data, if the perioperative complication rate exceeds 3%, the potential benefits of surgery are nullified.
Endarterectomy Versus Endovascular Treatment
The effectiveness of endarterectomy has been widely demonstrated in large-scale studies throughout the world but the studies lack validation in those individuals considered at high surgical risk. In such patients, endovascular treatment is an attractive alternative. As endovascular techniques and prosthetic material were being developed, as well as neuroprotection measures, postoperative care studies (characterized by a heterogeneity of the patients enrolled as well as the techniques used) comparing both treatment modalities were performed. Some studies viewed surgical endarterectomy as the gold standard, while others found that endovascular treatment had the same or better results than surgery. An evidence-based medicine analysis by the Cochrane group of five trials involving a total of 1269 patients concluded that to date there is no reason to change current recommendations to perform endarterectomy. Moreover, the use of endovascular techniques should be limited to clinical trials to date.
42.6 Cerebral Infarction
When cerebral circulation is suddenly interrupted and causes tissue necrosis, we speak of cerebral infarction. Several subtypes are distinguished; therefore, the following aspects should be considered in the evaluation of stroke patients:
According to the underlying pathophysiological mechanisms, different entities are recognized:
- Atherothrombotic infarction. Large (>1.5 cm in diameter) usually compromising the cortical, subcortical, carotid or vertebrobasilar territory. Diagnosis requires the presence of risk factors and previous generalized vascular compromise (peripheral arterial disease, ischemic heart disease, previous TIA, etc.) or vascular compromise demonstrated on further imaging studies such as Doppler, angiography, etc. Cardioembolic source should be excluded.
- Cardioembolic infarction. Usually cortical, moderate to large in size, with acute onset. Maximum neurological deficit from the beginning. TIA in several territories supports the diagnosis. Embolic sources (echocardiogram) and lack of atherothrombosis in large arteries must be demonstrated.
- Lacunar or small vessel infarction. Deep brain injury or small injury in the brainstem (<1.5 cm in diameter). Arterial hypertension and diabetes support the diagnosis. Microatheromatosis and lipohyalinosis are characteristics in anatomopathology.
- Infarction of undetermined etiology or “cryptogenic”. Features similar to atherothrombotic, two or more etiologies can coexist. Diagnosis is made by exclusion after checking the most common causes of infarction.
- Infarction of unusual cause. Variable size, without predilection for any vascular territory. It usually develops in individuals without classcrisk factors for cerebrovascular disease. It may be the beginning of a disease or occur during its course, e.g., vasculitis associated with connective tissue diseases (systemic lupus erythematosus, etc.), infections (endocarditis, pneumococcal meningitis, etc.), cancer, etc. Other causes: traumatic arterial dissection, complicated migraine, etc.
According to evolutionary profile:
- Formed or established infarction. When clinical manifestations occur from the beginning without worsening or improvement. An infarction can be considered established in a carotid territory if the neurological deficit remains changed after 24 hours, while the time period is extended to 72 hours for infarctions involving the vertebrobasilar territory.
- Progressive or evolving infarction. Worsening of the initial clinical features with either deepening of the deficit or the occurrence of new symptoms or both.
- Infarction with trend toward improvement. Follows a regressive course;usually recovery rate is near to 80% within 3 weeks.
According to the compromised vascular territory, we propose the draft of the classification of stroke in the community of Oxfordshire:
- Total Anterior Circulation Infarction (TACI). The following criteria must be met:
- Cortical dysfunction or dysfunction of higher brain functions such as aphasia, acalculia or visuospatial impairment.
- Motor and/or sensory disorders in at least two of the following areas: face, upper limbs, lower limbs.
- Homonymous hemianopsia.
- Cortical dysfunction or dysfunction of higher brain functions such as aphasia, acalculia or visuospatial impairment.
- Partial Anterior Circulation Infarction (PACI). When some of the following criteria are present:
- Cortical dysfunction or dysfunction of higher brain functions.
- Two TACI criteria.
- Motor and/or sensory deficit narrower than the LACI (e.g., deficit in only one limb).
- Cortical dysfunction or dysfunction of higher brain functions.
- Lacunar Infarction (LACI). This subtype doesn’t affect higher brain functions or cause hemianopsia. One or more of the following signs or symptoms may be present:
- Pure motor syndrome affecting at least two of the three body parts (face, arm, and leg).
- Pure sensory syndrome affecting two of the three body parts.
- Sensory-motor syndrome compromising two of the three body parts.
- Ipsilateral ataxic hemiparesis.
- Clumsy hand.
- Focal and acute abnormal movements.
- Pure motor syndrome affecting at least two of the three body parts (face, arm, and leg).
- Posterior Circulation Infarction (POCI). Diagnosed when one of the following events is present:
- Ipsilateral involvement of cranial nerves with contralateral motor and/or sensory deficit.
- Bilateral motor and/or sensory deficit.
- Oculomotor pathology.
- Cerebellar dysfunction.
- Isolated homonymous hemianopia.
- Ipsilateral involvement of cranial nerves with contralateral motor and/or sensory deficit.
42.6.1 Role of Neuroimaging in Ischemic Stroke
Imaging is essential in the initial evaluation of stroke victims. It is virtually impossible to differentiate ischemia from bleeding based purely on clinical medicine. CT without contrast material is the initial method of choice for diagnosis of a patient with acute neurological deficit, since it is available in most emergency units, can be performed quickly, and is the best method for identifying acute hemorrhage. It can also be performed in critically ill patients and patients under multiple monitoring devices or mechanical ventilation. Diagnosis of ischemic stroke is more difficult in the first 12 hours. Characteristics of the infarction in CT scan are a decrease in attenuation of parenchyma or hypodensity of about 10 to 30 HU, however, it may not be visible for until 24 hours after stroke onset. Only 5% of cerebral infarctions remain isodense during their evolution.
It is essential to investigate for the “early signs of infarction” visible in the first 6 hours in 85% of patients with ischemia of the middle cerebral artery. These signs can appear isolated or coexist with others.
They are:
- Spontaneously hyperdense sylvian artery (Figure 42.8).
- Blurring of the boundaries of the lenticular nucleus (Figure 42.9).
- Insular effacement (Figure 42.10).
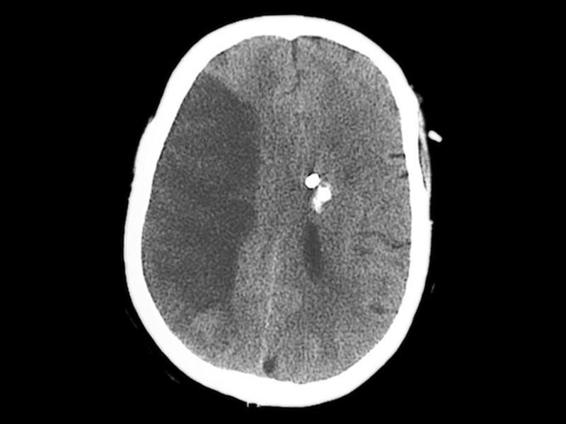
Figure 42.7. Structured sylvian infarction.
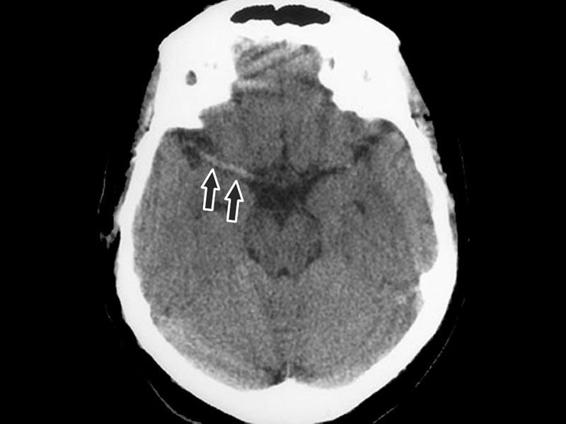
Figure 42.8. Sign: hyperdensity of the middle cerebral or sylvian artery.
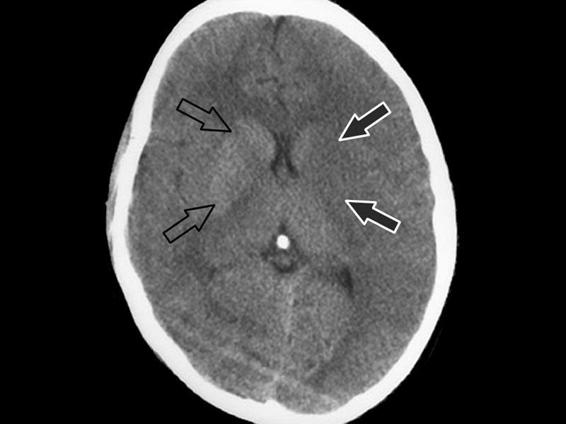
Figure 42.9. Blurring of the boundaries of the lenticular nucleus.
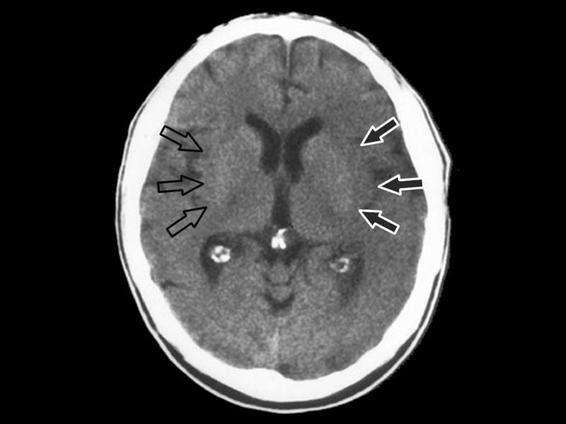
Figure 42.10. Effacement of the insular ribbon.
Cerebral edema manifested by effacement of sulci, ventricular distortion, deviation of the midline, and blurring of the boundary between the gray and white matter (Figure 42.11).

Figure 42.11. Early signs of cerebral edema (effacement of cortical sulci, ventricular distortion, absence of differentiation between the gray and white matter, midline deviation).
Valuable when present, these signs are not often seen in the initial study and their absence does not necessarily rule out ischemic infarction. They allow for the selection of patients eligible for thrombolytic therapy.
Magnetic resonance imaging is very useful in the evaluation of acute stroke. T1 and T2 sequences identify acute ischemia in an earlier stage compared with CT. The development of new techniques such as diffusion (diffusion-weighted imaging) and perfusion seem to be more sensitive and specific in the early hours.
The evaluation of images obtained by the diffusion imaging technique helps the clinician to identify 98% of infarctions in the early hours and is very effective in differentiating new ischemic strokes from existing ones. The combination of both techniques (diffusion and perfusion) may provide prognostic information to differentiate ischemic brain tissue from tissue with irreversible damage.
The comparative disadvantages of MRI versus CT are that MRI takes longer to perform, is not always available in all hospitals, is susceptible to patient motion artifacts, and is contraindicated in patients with pacemakers, intracranial surgical clips and very obese patients.
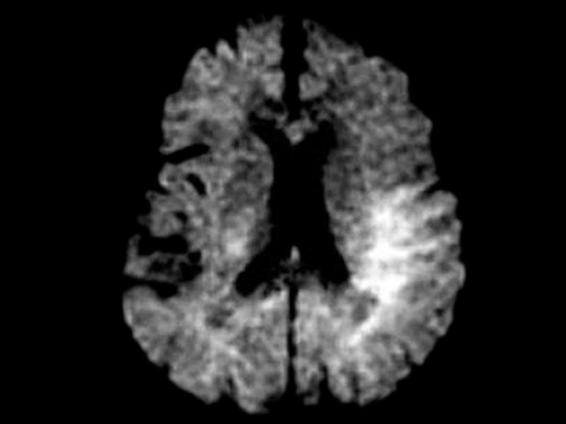
Figure 42.12. MR scan obtained with diffusion-weighted imaging.
42.6.2 Clinical Assessment Scales
Assessment scales are useful tools to reliably and objectively quantify the severity of stroke, its evolution, the final result, and when evaluating the benefit of a therapeutic measure.
Neurological scales allow us to detect deterioration or improvement in basic neurological functions; they must be applied systematically on admission and at set intervals at follow-up. The best one for the evaluation of patients in stupor or coma is the Glasgow Coma Scale (GCS), which was initially designed for assessing traumatic brain injury (TBI) and not for stroke. Among the neurological scales specific for stroke, the most widespread in Latin America is the scale devised by the US National Institutes of Health (NIH Stroke Scale). Other neurological assessment scales for stroke are the Scandinavian or Canadian neurological scales (www.strokeassociation.org).
42.6.3 Acute Phase Treatment
The best therapeutic results are unquestionably obtained with early recognition and diagnosis of the situation. The “nihilistic” concept must be set aside to make way for a strategy to shorten times, as aptly summarized with the concept “Time is brain.” Furthermore, efforts should focus on educating the general population and health professionals in the early identification of stroke symptoms to minimize the time of arrival at a specialized centre where these patients can be treated.
Cerebral resuscitation, together with clinical assessment and initial treatment, should begin without delay, as the final results largely depend on the speed with which this true medical emergency is approached.
42.6.4 Prehospital Phase
Depending on infrastructure facilities and human resources available, the initial steps should be directed to the diagnosis of the situation. Clinical examination is essential, because the patient is often unable to communicate, minimizes symptoms or reports them as simple and unspecific malaise. In the United States, abbreviated neurological assessment scales, such as the Cincinnati or Los Angeles scales, have been developed for use by paramedics assisting patients in the prehospital phase. Paramedics with basic training have demonstrated a sensitivity of about 62% in the detection of stroke, which increases to 90% on average when they have received trained in using a rating scale.
In Argentina, as in many other Latin American countries, prehospital emergency services have a doctor on board, providing a unique opportunity to achieve the established objectives.
The next step – perhaps the most important – is to establish the “time zero or time of onset of symptoms.” If the patient is “awake” with symptoms of stroke, time zero is when the patient was seen “normal” for the last time.
During transfer, support is the same as in any medical emergency. The ABC rules are followed, i.e. ensuring airway patency and ventilation, monitoring cardiovascular and neurological status, as well as checking blood glucose levels if possible.
Stroke patients may have an altered state of consciousness and reduced cough and deglutition reflexes: the risk of aspiration and airway obstruction due to relaxation of the laryngopharyngeal muscles makes them particularly predisposed to the development of such secondary insults such as hypoxemia or hypercapnia.
Oxygen should be administered when arterial oxygen saturation monitoring is not available or when <92%.
42.6.5 Evaluation in the Emergency Room
Upon arrival at the hospital, monitoring of vital signs and ABC measures will continue. In the meantime, preferably two venous accesses will be placed, samples taken for blood tests, and chest radiograph and an electrocardiogram should be obtained. The latter can identify acute myocardial infarction or arrhythmias predisposing to stroke, such as atrial fibrillation. Beginning infusion of isotonic fluids (normal saline); and if present, hypoglycemia will be quickly treated, oxygen administration will continue and the stroke team recluted. Neurological status is will be assessed with a scale such as NIH Stroke scale. A CT scan will be obtained after clinical stabilization. Every effort should be made to ensure that all the above measures, including analysis of the images, take no longer than 45 minutes. The management of arterial hypertension, controversial and debated, is considered in detail below. Here, we mention only the formal contraindication to the use of vasodilators such as sublingual nifedipine as an abrupt drop in pressure levels may increase the extent of infarction. If CT detects no bleeding, ischemic stroke is diagnosed, and if the patient is in the window period, i.e., within 3 hours since the onset of symptoms, thrombolytic therapy should be considered.
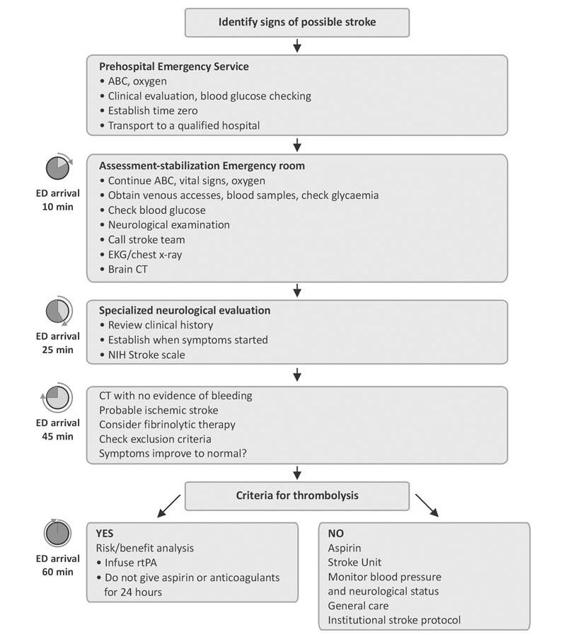
Figure 42.13. Algorithm for the management of patients with suspected stroke.
rtPA = recombinant tissue plasminogen activator.
42.6.6 Stroke Units or Neurointensive Care Unit
Stroke units were first developed in North America in the 1960s in the wake of the success of coronary care units. Basically, a stroke unit is a section of a hospital where stroke patients are treated by a multidisciplinary team with a special expertise in stroke, including physicians, nurses, physiotherapists, occupational therapists, speech therapists and social workers.
Recent studies show an 18% relative decrease in mortality, a 3% absolute reduction in mortality and in the need for home care. Additional benefits were obtained with a 6% increase in the number of surviving patients able to perform activities of daily living.
Minimum requirements | Additional tests (performed according to physician’s opinion) |
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



