Chapter 15 Until recently, the only medical therapy approved by the Food and Drug Administration (FDA) for acute stroke treatment was intravenous (IV) recombinant tissue plasminogen activator (tPA) administered within 3 hours of symptom onset for patients eligible for thrombolysis1,2 (Table 15.1). The new American Heart Association/American Stroke Association (AHA/ASA) guidelines1 recommend evaluating patients for IV tPA 3 to 4.5 hours after stroke symptom onset using the same eligibility criteria as the 0- to 3-hour time window along with any one of the additional exclusion criteria shown in Table 15.1 (based on data from the European Cooperative Acute Stroke Study [ECASS] III2). Patients who do not meet the eligibility criteria for intravenous thrombolytic (IVT) therapy, who fail to improve neurologically after thrombolytic therapy, or who improve and then worsen (patients with reocclusion) are candidates for endovascular revascularization therapies. At our center, 94 patients with a mean presentation National Institutes of Health Stroke Scale (NIHSS) score of 14.7 were treated by endovascular interventions within 3 hours of stroke symptom onset.3 In all these patients, tPA IVT was contraindicated or had failed. Partial to complete recanalization (Thrombolysis in Myocardial Infarction [TIMI] score of 2 or 3) was achieved in 62 of 89 (70%) patients presenting with significant occlusion (TIMI 0 or 1). Postprocedure symptomatic intracranial hemorrhage (SICH) occurred in five patients (5.3%), which was purely subarachnoid hemorrhage in three of these patients. The total mortality rate, including procedural mortality, progression of disease, or other comorbidities, was 26.6%. Overall, 36.7% of patients had a modified Rankin scale score (mRS) of ≤2 at discharge. Mean NIHSS at discharge was 6.5, representing an overall 8-point improvement in NIHSS score. In the Mechanical Embolus Removal in Cerebral Ischemia (MERCI),4 Multi MERCI,5 and the combined analysis of Interventional Management of Stroke (IMS) I and II Studies,6 functional outcome (measured by mRS score of ≤2 at 3 months) was significantly better and the 3-month mortality was significantly lower in patients who had TIMI 2 or 3 recanalization than in patients in whom vessels failed to recanalize after endovascular therapy. Rha and Saver7 reviewed 53 studies including 2,066 patients and found that good functional outcomes (mRS score ≤2) at 3 months were more frequent in patients with vessel recanalization than without vessel recanalization. The 3-month mortality rate was reduced in patients whose vessels were recanalized. Higher rates of recanalization were achieved with endovascular methods, particularly mechanical therapies, and consequently, were associated with better outcomes. The obvious advantages of computed tomography (CT) over other penumbral imaging techniques are its ubiquitous availability, speed of imaging, cost-effectiveness, and accessibility in the emergency department. We use a combined multimodal CT stroke protocol consisting of noncontrast CT (NCCT), CT perfusion (CTP), and CT angiography (CTA) to select patients for endovascular thrombolysis. Other groups8–10 have similarly noted benefits of combined CTA and CTP imaging in rapid assessment of acute stroke. CTP imaging is helpful in evaluating the ischemic core [very low cerebral blood flow (CBF; > 70% reduction), very low cerebral blood volume (CBV; <2 mL/100 g),11 and extremely prolonged transit time].12–14 In our experience, patients with large ischemic cores and even small ischemic cores in the basal ganglia region have a high risk for SICH and poor outcome. We try to avoid endovascular thrombolysis in these patients; and, if compelled to intervene due to the presence of a large penumbra, avoid pharmacologic thrombolysis and glycoprotein (GP) IIb/IIIa antagonists. The disadvantages of CTP are radiation exposure, incomplete validation, and qualitative differences in postprocessing software.
Acute Stroke Revascularization
 Background
Background
 Patients Who Are Not Candidates for Intravenous Thrombolysis or in Whom It Fails
Patients Who Are Not Candidates for Intravenous Thrombolysis or in Whom It Fails
 Endovascular Therapy Leads to Higher Recanalization Rates and Improved Outcomes
Endovascular Therapy Leads to Higher Recanalization Rates and Improved Outcomes
 Multimodal Computed Tomographic Imaging to Assess the Ischemic Penumbra (The Therapeutic Target)
Multimodal Computed Tomographic Imaging to Assess the Ischemic Penumbra (The Therapeutic Target)
For Patients Presenting within the 3-Hour Time Window before Beginning Treatment |
Diagnosis of ischemic stroke causing measurable neurologic deficit |
Neurologic signs not clearing spontaneously |
Neurologic signs not minor and isolated |
Caution should be exercised in treating a patient with major deficits |
Stroke symptoms should not be suggestive of SAH |
No head trauma or stroke in the previous 3 months |
No myocardial infarction in the previous 3 months |
No gastrointestinal or urinary tract hemorrhage in the previous 21 days |
No major surgery in the previous 14 days |
No arterial puncture at a noncompressible site in the previous 7 days |
No previous ICH |
Blood pressure not elevated (systolic <185 mm Hg; diastolic <110 mm Hg) |
No evidence of active bleeding or acute trauma (fracture) |
Not taking an oral anticoagulant or, if anticoagulant being taken, international normalized ratio ≤1 |
If receiving heparin in previous 48 hours, activated partial thromboplastin time must be within normal range |
Platelet count ≥100,000 mm3 |
Blood glucose concentration ≥50 mg/dL (2.7 mmol/L) |
No seizure with postictal residual neurologic impairment |
No multilobar infarction seen on NCCT scan (hypodensity >1/3 cerebral hemisphere) |
Patient or family members understand the potential risks and benefits from treatment |
Additional Eligibility Criteria for Patients Presenting between 3 and 4.5 Hours after Onset |
≤80 years |
If taking oral anticoagulants, international normalized ratio <1.7 |
Baseline NIHSS score ≤25 |
No history of both stroke and diabetes |
Abbreviations: ICH, intracranial hemorrhage; NCCT, noncontrast computed tomographic scan; NIHSS, National Institutes of Health Stroke Scale; SAH, subarachnoid hemorrhage
Source: del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A Science Advisory from the American Heart Association/American Stroke Association. Stroke 2009; 40:2945– 2948; and Adams HP, Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711.
 Patient Selection and Complication Avoidance
Patient Selection and Complication Avoidance
Better patient selection decreases complications, including SICH. The three major criteria for selection of a patient for endovascular thrombolysis are (1) contraindication for IV tPA or neurologic condition that did not improve or worsened after initial improvement with IV tPA; (2) time window from stroke symptom onset (as discussed above); and (3) ischemic penumbra – the therapeutic target (as discussed above). On the basis of these concepts and our experience, mechanical revascularization therapy is the first choice at our center for patients who present after 3 hours of stroke symptom onset and patients with wake-up strokes (in whom the time of stroke symptom onset is unknown) after evaluating and confirming the presence of large vessel occlusion. Intraarterial pharmacologic thrombolysis is used only if the site of occlusion is distal and not reachable for mechanical therapy or as an adjunct to treat distal embolization should it occur after mechanical therapy.
Although IV tPA between 3 and 4.5 hours after stroke symptom onset has been endorsed by the recent AHA/ASA guidelines,1 we believe that all patients with acute ischemic stroke should be evaluated for intraarterial therapies. At our center, patients who present up to 4.5 hours after stroke symptom onset with a large vessel occlusion and have no risks of SICH by clinical, radiologic, and physiologic data are candidates for bridging therapy (IVT and intraarterial thrombolysis [IAT]). Bridging therapy allows a full IV tPA dose to be initiated; and if there is no significant improvement at 30 minutes, the patient is prepared for intraarterial therapy. For those patients who are at higher risk of SICH on the basis of CTP criteria, irrespective of the time window of presentation, mechanical thrombolysis can be considered; and we defer using pharmacologic thrombolysis in these patients to the attending stroke neurologist.
Our center’s current protocol for selection of treatment strategies and posttreatment management after revascularization of acute ischemic stroke patients is shown in Table 15.2. It is important to remember that CTP is only one of the risk-assessment tools for decision-making in these patients. The final decision to revascularize and the choice of thrombolytic modality are made after integrating the CTP findings with the clinical presentation and patient factors and a discussion that involves the neurointerventionist and the stroke neurologist.
All patients with a clinical diagnosis of acute ischemic stroke undergo multimodal CT stroke imaging that includes cranial NCCT, CTA from the aortic arch through the cranial vertex, and CTP whole-brain studies. |
After hemorrhagic stroke is ruled out, patients with large vessel occlusion (cervical ICA, petrous ICA, intracranial ICA, MCA-M1, MCA-M2, A1, intracranial VA, BA, PCA-P1, PCA-P2) and/or with contraindications for IVT are assessed for endovascular revascularization. |
Patients who have a high risk for SICH – (1) ischemic core in the basal ganglia region, and/or (2) large cortical or subcortical ischemic core on CTP (>50% of at-risk territory) – are identified. |
Patients who have known stroke symptom onset, are within 4.5 hours of stroke onset, and are not at high risk for SICH are evaluated for IVT. |
Patients with large vessel occlusion, at low risk for SICH, and within the 4.5-hour time window have bridging therapy (IVT + endovascular revascularization). |
Patients who do not improve at least 4 points on the NIHSS after IVT or improve and then deteriorate after IVT are again evaluated for endovascular revascularization after a repeat cranial NCCT to exclude ICH. |
Patients who are candidates for endovascular revascularization are carefully selected for angiography after weighing the benefit of reperfusion with the risk of SICH based on multimodal CT stroke imaging findings. |
All patients who are selected for intervention on the basis of angiographic results receive sufficient heparin to maintain the activated coagulation time between 250 and 320 seconds for the duration of the procedure. Patients who are not taking aspirin or clopidogrel (or ticlopidine) are treated with aspirin (325 mg; enteric-coated, if necessary) immediately before the procedure. |
Mechanical revascularization therapies – wire manipulation, Merci device (Concentric Medical, Mountain View, CA), Penumbra device (Penumbra Inc., Alameda, CA) – are the primary options for endovascular recanalization. |
IAT with t-PA is used for occlusions that are not reachable with current devices or as an adjunct after mechanical revascularization for distal emboli (only in patients with low risk of SICH). |
Wingspan (Boston Scientific, Natick, MA) or Enterprise (Codman Neurovascular, Raynham, MA) stent placement is used as a bailout for patients in whom vessels cannot be recanalized with current FDA-approved modalities and who have an occlusion at a site that can be stented under Humanitarian Device Exemption and FDA-approved trials. Patients considered for stent placement are given a loading dose of either clopidogrel (600 mg) or ticlopidine (1 g) and aspirin 650 mg. |
GP IIb/IIIa inhibitors are used only if intraluminal thrombus formation is seen after endovascular recanalization. |
Patients receiving stents are placed on dual antiplatelet therapy for 3 months and aspirin for life. All other patients with acute ischemic stroke are maintained on aspirin for life. |
Patients are observed in the intensive care unit for 12–24 hours after treatment, and a blood pressure of ∼150/90 mm Hg is maintained to avoid reperfusion injury. |
All patients have standardized rehabilitation and disposition methods determined by the same stroke neurology team. |
All patients are placed on a standardized risk modification regimen that includes control of hypertension, diabetes mellitus, and hyperlipidemia; an exercise program; smoking cessation; and treatment for obesity. |
Abbreviations: AHA/ASA, American Heart Association/American Stroke Association; BA, basilar artery; CT, computed tomography; CTA, computed tomographic angiography; CTP, computed tomographic perfusion; FDA, Food and Drug Administration; GP, glycoprotein; IA, intraarterial, IAT, intraarterial thrombolysis; ICA, internal carotid artery; IV, intravenous; IVT, intravenous thrombolysis; MCA, middle cerebral artery; mRS, modified Rankin Scale; NCCT, noncontrast CT scan; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery; SICH, symptomatic intracranial hemorrhage; tPA, tissue plasminogen activator; VA, vertebral artery
 Technique
Technique
Mechanical Thrombolysis or Embolectomy
Mechanical recanalization systems can be divided into two major groups, proximal or distal devices, according to where they apply force on the thrombus. Proximal devices apply force to the proximal base of the thrombus. This group includes various aspiration catheters. Distal devices approach the thrombus proximally, but then are advanced by the guidewire and microcatheter across the thrombus to be unsheathed distally, where force is applied to the distal base of the thrombus. This group includes snare-, basket-, and coil-like devices. In an animal model,15 proximal devices were faster in application and associated with a low complication rate. The distal devices were more successful at removing thrombotic material, but their method of application and attendant thrombus compaction increased the risk of thromboembolic events and vasospasm.16,17 Advantages and disadvantages of mechanical revascularization strategies overall are summarized in Table 15.3.18 The current FDA- approved embolectomy devices are the Penumbra (proximal device; Penumbra Inc., Alameda, CA) and the Merci retriever (distal device; Concentric Medical Inc., Mountain View, CA).
Advantages |
Devices used for mechanical thrombolysis or embolectomy lessen and may even preclude the use of pharmacologic thrombolytics, thus reducing the incidence of SICH. |
These devices may extend the treatment window beyond 6–8 hours from onset. |
Mechanical fragmentation of the clot increases the surface area of clot available for endogenous and exogenous fibrinolysis. |
Recanalization time may be faster. |
Devices used for mechanical thrombolysis may be effective for thrombi or other material resistant to thrombolytics that occlude the vessel. |
Mechanical thrombolysis has emerged as the key option for patients who have a contraindication for pharmacologic thrombolysis, such as recent surgery or abnormal hemostasis,18 or have a late presentation.4,5,20 |
Disadvantages |
Technical difficulty of navigating mechanical devices through the tortuous intracranial vasculature |
Excessive trauma to the vasculature |
Distal embolization from fragmented thrombus |
Abbreviations: SICH, symptomatic intracranial hemorrhage
Microguidewire Manipulation and Snare
The most common method for mechanical thrombus disruption is probing the thrombus with a microguidewire. This technique appears to be useful in facilitating pharmacologic thrombolysis.19
Merci Device
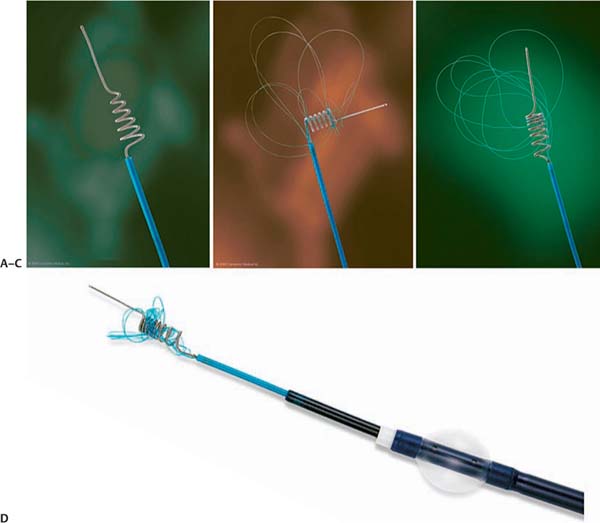
The Merci retrieval system is a shaped wire constructed of nitinol. The flexible corkscrew-like tip can easily be delivered through a microcatheter into the vessel distal to the occlusion site. When deployed, this device returns to its pre-formed coiled shape to ensnare the thrombus. The thrombus is bypassed and the retriever deployed from inside the catheter distal to the thrombus. The corkscrew-like tip is pulled back slowly to ensnare the clot as a corkscrew would ensnare a cork. The retriever is then retracted into the guide catheter under proximal flow arrest. different versions of this device are available (Fig. 15.1): in the first-generation devices (X5 and X6), the nitinol wire was shaped in helical-tapering coil loops. The second-generation devices (L4, L5, and L6) differ from the X devices by the inclusion of a system of arcading filaments attached to a nontapering helical nitinol coil, which has a 90-degree angle in relation to the proximal wire component. The third-generation devices (V series) have variable pitch loops under a linear configuration with attached filaments. The retriever device is deployed through a 2.4F microcatheter (14X or 18L, Concentric Medical). The recent addition of a 4.3F distal access catheter has provided additional coaxial support to the system, resulting in improved deliverability with the potential for simultaneous thromboaspiration as well. Merci devices are available in various diameters from 1.5 to 3 mm, depending on the caliber of the occluded vessel. An illustrative case of Merci retrieval is provided in Fig. 15.2.
Merci devices are regularly used in combination with proximal balloon occlusion in the internal carotid artery (ICA), in addition to aspiration from the guiding catheter, to reduce the risk of distal thromboembolism. In general, an 8F to 9F sheath and balloon catheter of similar size are used. After placement of the balloon catheter in the ICA, a microcatheter, in combination with a microwire, is navigated to the occlusion site. This catheter is then advanced beyond the thrombus. An injection of contrast material distal to the thrombus is recommended to estimate the length of the occlusion and illustrate the anatomy of the distal vessel. The device is then introduced into the microcatheter and unsheathed behind the thrombus. The balloon at the tip of the guiding catheter is inflated. During slow retraction of the device and mobilization of the thrombus, aspiration is applied at the guiding catheter. The device and thrombus are retrieved into the guiding catheter, and the balloon is deflated. In clinical practice, the entire procedure often has to be repeated multiple times to recanalize the vessel. Furthermore, the application of the balloon catheter might be limited in cases of high-grade ICA stenosis.
Fig. 15.1 Types of Merci devices. (A) Type X. (B) Type L. (C) Type V. (D) Catheter system. (Courtesy of Concentric Medical, Mountain View, CA).
The Merci device often requires three or four passes before flow is restored. This process delays the time to recanalization in these patients. The aforementioned new distal access catheter available for use with the Merci device allows placement of smaller triple coaxial guide catheter near the lesion of interest for repeated quick access to the lesion and to transmit the traction force better in a straighter angle at a shorter distance.
FDA approval of the Merci device in 2004 was based on a review of data obtained in the multicenter Merci Trial that involved 141 patients (mean age 60 years; mean NIHSS score 20) ineligible for standard thrombolytic therapy.4,20 The Multi-MERCI Trial5 was a prospective, multicenter, single-arm registry that included 164 patients (mean age 68 years; mean NIHSS score 19) treated with different Merci retrieval systems (X5, X6, and L5). Patients with persistent large vessel occlusion after IVT (with tPA) were also included in the study, and adjunctive IAT (using tPA) was allowed. Use of the Merci device has also increased recanalization rates with intracranial ICA occlusion.21 Two ongoing prospective randomized trials are using the device, the MR and Recanalization of Stroke Clots Using Embolectomy Trial (MR Rescue) and the IMS III Trial.22
Penumbra Device
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree