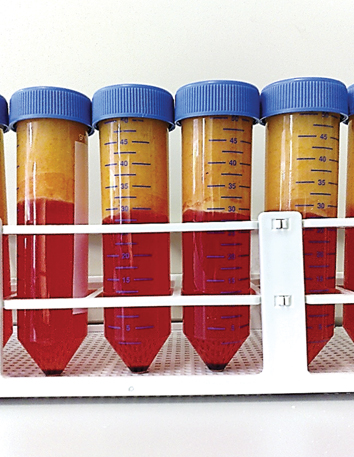
Fig. 8.1
Unprocessed lipoaspirate showing semi-liquid fat on the top (yellow) with blood and infiltration fluid in the lower layer (red)
8.3.2 Processing of Adipose Tissue
When lipoaspirate is transported to the lab, it travels in the original surgical waste container at room temperature. A block of excised tissue would routinely be transported to the laboratory wrapped in saline-soaked gauze to prevent desiccation and secured in a sterile, screw top container.
Our method for ASC isolation is based on the seminal paper by Zuk and colleagues from 2001 with minor modifications (Zuk et al. 2001). Following isolation, ASC are contained within the stromal vascular fraction (SVF) cell pellet in the bottom of the tube (see Fig. 8.3 and Fig. 8.4). Cryopreservation of the SVF results in red blood cell (RBC) lysis so we rarely perform a specific RBC lysis step, preferring instead to repeat the initial wash step to decrease the RBC load if necessary. However, if you wish to count the cells in the SVF directly then it is likely that you will need to perform a RBC lysis.
1.
Excised fat is placed in a sterile plastic tray with a small amount of Complete medium. Using sterile forceps and a pair of sterile scissors, or scalpel blade, it is cut into small pieces (ideally less than 5 mm by 5 mm) to facilitate digestion. These pieces are then placed into a 50 ml tube for processing as per lipoaspirate. This step is not required if semi-liquid lipoaspirate is used.
2.
Top layer of lipoaspirate, containing adipose tissue (or excised and diced adipose tissue), is placed into 50 mL tubes, ensuring the tubes are no more than half full of lipoaspirate.
3.
Tubes are filled to 50 mL with Complete medium, agitated to mix and centrifuged at 700 g for 10 min.
4.
Floating adipose tissue is transferred into fresh 50 ml tubes and the liquid lower layer is discarded (see Fig. 8.2).

Fig. 8.2
Washed lipoaspirate showing the top layer of floating adipose tissue to be retained (yellow) and lower liquid layer to be discarded (red)
5.
Repeat steps 2–4.
6.
An equal volume of 0.075 % Collagenase type I in PBS is added to each tube of washed lipoaspirate for digestion.
7.
Tubes are placed in a warm water bath for 1 h for digestion. An agitating water bath is ideal for digestion. If not available, a normal water bath may be used but the tubes should be inverted several times or agitated by hand every five to ten minutes during the digestion process.
8.
Tubes are centrifuged at 700 g for 10 min.
9.
Supernatants containing floating adipocytes and media are discarded. Cell pellets are resuspended in Complete medium.
10.
Resuspended cells are passed through a 100 μm cell filter into a clean 50 mL tube to remove connective tissue.
SVF is now ready for cryopreservation or culture.

Fig. 8.3
Lipoaspirate after collagenase digestion and spin cycle, showing floating mature adipocytes in the top layer (yellow), media in the lower layer (orange) and a cell pellet at base of tube

Fig. 8.4
Close up of SVF pellet after digestion and spin cycle
8.3.3 Culture of Isolated Cells
1.
A cell count is performed on fresh SVF or recovered cells using Trypan blue to identify non-viable cells.
2.
1 × 106 cells are seeded into each T75 flask with 12 mL of Complete medium.
3.




Cells are cultured at 37 °C/5 % CO2 in a humidified atmosphere.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








