Advances in the Management of Hypervascular Tumors of the Spine
A. Hadjipavlou
K. Kakavelakis
E. Pateromichelakis
P. Katonis
INTRODUCTION
Tumors of the spine are more difficult to manage than their counterparts in the appendicular skeleton, mostly because they are located in areas that are difficult to access, often in close proximity to vital neurovascular elements.
Optimal surgical management of spinal tumors consists of decompression of neural elements with wide excision of the tumor mass, reconstruction of spinal structures, and stabilization with instrumentation.
The most commonly encountered hypervascular tumors in the spine are (a) primary tumors, which include hemangiomas, aneurysmal bone cysts, osteoblastomas, and hemangioendotheliosarcomas; and (b) metastatic tumors, which include renal cell carcinomas, hepatoma, hypervascular pheochromocytomas, and infrequently, certain types of adenocarcinomas, multiple myelomas, and cancers of the thyroid.
Hypervascular tumors of the spine are extremely challenging. Profuse intraoperative bleeding usually interferes with radical excision of the tumor; surgery may even have to be aborted. This in turn potentially results in compromised outcomes with high morbidity and mortality. For this reason, several modalities have been suggested as an adjunct or as an alternative to surgery. These procedures include cell saver, radiation therapy, transarterial embolization, intralesional injections of various substances, vertebral cement augmentation through vertebroplasty or kyphoplasty techniques, thermal ablation (cryotherapy, etc.), and combinations of various modalities. Each of these procedures has its advantages and its disadvantages.
Cell Saver
Cell saver can be helpful in some cases but is not optimal in patients with malignant tumors to save blood and minimize transfusions. Reinfusion of irradiated red blood cells is a possible solution (40). However, this does not prevent intraoperative bleeding that interferes with surgery.
Radiation Therapy
According to Tong et al. (89) and Ratanatharatorn et al. (72), who reviewed the existing literature on the outcomes of radiation, the reports are not satisfactory. The overall effectiveness rates were 54% for complete response and was obtained in only 50% of patients. The relapse rate of the patients who survived at least 12 weeks was 30%.
Radiation therapy can reduce the vascularity of hypervascular tumors, but it is not an ideal alternative in their management; the response is often neither immediate nor effective. Furthermore, healing can be compromised, predisposing to infections. Treating benign lesions with radiotherapy is controversial because of the unacceptable risk of severe complications. Reported complications include postradiation myelopathy, radiation-induced sarcoma, and possible growth disturbance in children (30,51,52,69,76,83). Papagelopoulos et al. (69) reported a case of postradiation osteosarcoma that occurred 7 years after radiation for a spinal aneurysmal bone cyst and that resulted in death of the patient.
Some promote radiation therapy as the treatment of choice for vertebral hemangiomas, particularly for multiple spinal involvement (10,23). Vertebral hemangiomas are sensitive to radiation and respond to doses of 2,000 to 4,000 cGy (37,57). However, the recommended dose of 2 Gy per day for several weeks precludes the immediate effectiveness of radiation therapy; therefore, radiation is not recommended in acute situations. Furthermore, no clear dose-response relation has been reported, and complete pain relief after irradiation ranges from 37% to 87.5% (4,93). Radiographic regression of the lesion is often minimal. A review article that included 21 reports of 63 cases of vertebral hemangiomas demonstrated that, overall, radiotherapy resulted in a complete remission of symptoms in 57% of cases, partial remission in 32%, and no response in 11% of cases (45). Radiation also has the potential risk of radionecrosis (37,62) and may induce sarcoma (54,67).
Transarterial Embolization
Transarterial embolization of spinal hypervascular tumors has been described as both a primary therapeutic measure (22,48) and an adjunct to surgical resection (12,28,61,68).
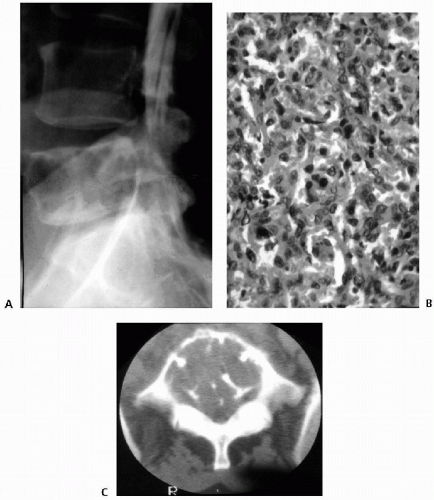
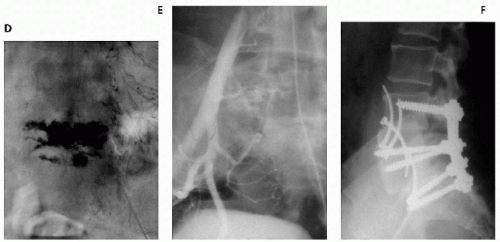
The goal of preoperative transarterial embolization is to improve perioperative blood loss to facilitate more-radical resectability of the tumor. Transarterial embolization has been promoted as a safe and effective procedure for treating hypervascular spinal tumors (Fig. 10.1). The most commonly used embolic materials are polyvinyl alcohol foam, gelfoam particles, absolute alcohol sponges, and metallic coils, as well combinations of these materials. Results of embolization largely depend on the materials used. Gelfoam particles have been used in early studies. Proximal gelfoam occlusion may augment the distal thrombosis within the tumor and produce a transient devascularization of the region adjacent to the tumor. However, proximal occlusion of large vessels without penetration into the tumor architecture results in ineffective embolization with very early recanalization through enzymatic resorption of the material. Rapid revascularization of the tumor was observed within 3 days from adjacent collaterals, resulting in considerable intraoperative bleeding (34). For this reason, surgery should be undertaken within 24 hours after gelfoam particle embolization. Polyvinyl alcohol, composed of nonresorbable particles of 150- to 250-mm, diameter allows relatively distal embolization at the capillary level (68). After polyvinyl alcohol particle embolization, the same arteries can be occluded proximally by using gelfoam. Absolute ethanol has also been successfully used for preoperative embolization of spinal metastases from renal cancer because of its deep penetration and intimal sclerosis with inflammatory reaction (85). However, the use of liquid embolization agents for spinal metastases has been associated with a high rate of neurologic complications (8,75). Additional use of coils after polyvinyl alcohol particle embolization has been reported to provide no further benefit (9).
After transarterial embolization, the expected average perioperative blood loss may range from 1.5 to 2.2 L in the lumbar and thoracic spine (11,34,53,68,73,82,84,85), as opposed to 5 to 15 L blood loss when preoperative embolization is not used (34,53,73).
In our series of 10 cases, in which a preoperative embolization alone was conducted, intraoperative estimated blood loss in excess of 3 L occurred in five cases. Mean estimated blood loss was 2.8 L. In one of our patients, bleeding was profuse (>8 L), and surgery was prematurely terminated, resulting in suboptimal tumor resection (61). The mean intraoperative blood loss after preoperative embolization in the cervical spine is 2.4 L (90). However, transarterial embolization of tumors in the cervical spine is technically more difficult and involves a higher risk of neurologic complications (18,23,27,47,73). Frequent anastomoses between the carotid, vertebral, and subclavian artery branches increase the risk of cerebral or spinal cord embolization, although ring anastomoses at the base of the brain allow occlusion of one of the main arteries entering the neurocranium (8,35,58,88). In several situations, successful embolization may not be possible. Embolization may be incomplete because of peculiarities of vascular anatomy or because embolization into vessels feeding the tumor may potentially compromise the vascularity of the spinal cord. The presence of an anterior spinal artery at the same pedicle is a contraindication for embolization (84). The untoward embolization of an anterior spinal artery may injure the pyramidal tracts and cause severe motor deficits (15). Although partial embolization seems to reduce blood loss in most patients, this may not be sufficient to prevent profuse bleeding during the procedure (53,73). Angiography may also fail to encounter feeding vessels that could be embolized (3). Finally, the watershed area of the mid-thoracic spine embolization may well be too dangerous in which to perform (86).
Surgical techniques for decreasing blood loss in tumors that are incompletely embolized involve adjacent-level segmental artery ligation and the use of enbloc rather than piecemeal resection.
Preoperative coil embolization has failed to show any significant effect on intraoperative blood loss in hypervascular spinal metastases (9), especially in metastases from renal cell carcinoma that may open collateral channels within hours.
Even technically successful and subjectively complete transarterial embolization may fail to eliminate intraoperative bleeding satisfactorily. Complete devascularization of a hypervascular tumor requires that precapillaries, capillaries, and even the proximal parts of the draining veins are occluded by depositing the embolic agents as deeply into the tumor as possible (55). Distribution of embolizing particles can be less than optimal when multiple feeders supply the microvascular tumor bed. Furthermore, tumors like metastatic renal cell carcinoma have irregular arterial feeders, widespread networks of arteriovenous shunts, and dilated capillaries, resulting in rapid circulation with early venous drainage (49,55). Furthermore after successful transarterial embolization, the remaining central tumor core can recruit previously minor arterial supply in response to the occlusion of its primary arterial feeders (79).
In conclusion, the overall reported outcomes of preoperative transarterial embolization are suboptimal. This has led to the use of alternative techniques such as intralesional injection of various agents, cryotherapy, and vertebral-body cement augmentation. These therapeutic interventions can be used as adjuncts to surgery or as stand-alone procedures.
Intralesional Injection of N-butyl cyanoacrylate or sclerosing agents [ethanol alcohol, bleomycin, OK-432 (Picibanil), Ethiblock]
Direct intralesional injection can be useful in tumors with a peculiar vascular anatomy or when embolization into the feeding vessels is not recommended (e.g., in watershed areas of the mid-thoracic spine, or failure to encounter the feeding vessels). Direct intralesional injection of a sclerosing agent has several advantages. The procedure is minimally invasive, can be performed under local anesthesia and intravenous sedation, and usually does not entail significant blood loss. Herbreteau et al. (44) reported a case of a metastasis located in the fourth cervical vertebra, and Chiras et al. (17) reported on a hypervascular pheochromocytoma metastasis of the sixth cervical vertebral body that were both successfully managed preoperatively by a direct intravertebral injection of an alcoholic embolizing emulsion. Cotten et al. (20) reported intralesional percutaneous injection of N-butyl cyanoacrylate, a rapidly polymerizing cyanoacrylate plastic, into the posterior arch to minimize blood loss during decompressive laminectomy in four patients with vertebral hemangiomas with neurologic compression. More recently, Schirmer et al. (79) reported five cases with metastatic renal cell carcinoma to the spine treated with a combination of transarterial embolization and direct percutaneous injection of N-butyl cyanoacrylate. They theorize that direct intralesional injection achieves closer to a true end-organ embolization at the level of the capillary bed than does the more proximal occlusion of the blood supply at the level of small arterioles that is usually achieved by transarterial embolization. The injected material assumes the form of the entire tumor, including a portion of the epidural component, without changing its volume, thus minimizing the risk of neurologic deficit. The embolized tumor maintains a soft consistency and can be readily removed in a piecemeal fashion. Intralesional embolization can effectively reduce tumor vascularity and result in decreased blood loss
compared with preoperative transarterial embolization. The estimated blood loss during surgery reported in the series of Schirmer ranged from 300 to 800 ml. However, a possible drawback to this technique is the risk of retrograde flow of embolic material through a feeder artery. Casasco et al. (14) described this complication when treating juvenile nasopharyngeal angiofibromas at the cranial base in two patients. They encountered a fatal polymerization of N-butyl cyanoacrylate in the middle cerebral artery in one patient, and embolization of a collateral of the ophthalmic artery in another patient, resulting in vision loss. For this reason, direct N-butyl cyanoacrylate injection should be avoided into any tumor receiving arterial supply from a vessel leading to a spinal or cerebral artery.
compared with preoperative transarterial embolization. The estimated blood loss during surgery reported in the series of Schirmer ranged from 300 to 800 ml. However, a possible drawback to this technique is the risk of retrograde flow of embolic material through a feeder artery. Casasco et al. (14) described this complication when treating juvenile nasopharyngeal angiofibromas at the cranial base in two patients. They encountered a fatal polymerization of N-butyl cyanoacrylate in the middle cerebral artery in one patient, and embolization of a collateral of the ophthalmic artery in another patient, resulting in vision loss. For this reason, direct N-butyl cyanoacrylate injection should be avoided into any tumor receiving arterial supply from a vessel leading to a spinal or cerebral artery.
Sclerosing agents are substances that cause marked tissue irritation and thrombosis with subsequent local inflammation and tissue necrosis. This inflammation and tissue necrosis result in fibrosis and tissue contraction. The most popular sclerosing agents are Ethibloc alcohol, bleomycin, and OK-432 (Picibanil).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree