Fig. 2.1
Marchiafava-Bignami disease depicted on CT. Axial non-contrast CT shows hypoattenuation in the genu of the corpus callosum (arrow) and adjacent periventricular white matter (arrowheads)
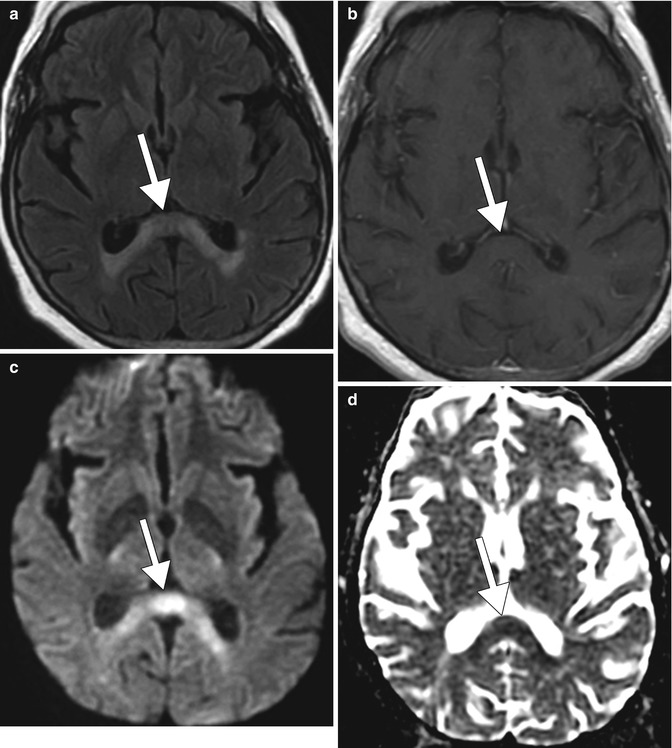
Fig. 2.2
Marchiafava-Bignami disease depicted on MRI. Axial FLAIR MRI (a), post-contrast T1-weighted MRI (b), DWI (c), and ADC map (d) show non-enhancing T2 hyperintensity in the splenium of the corpus callosum (arrows)
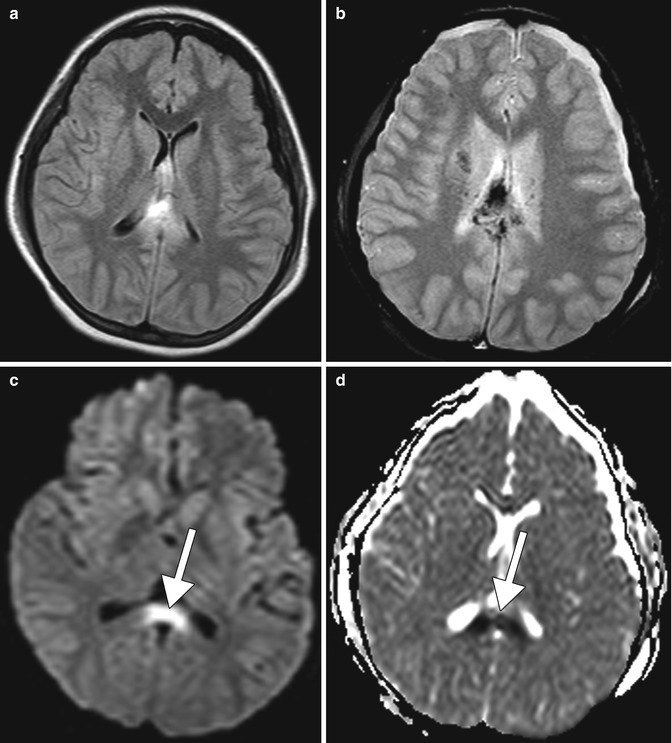
Fig. 2.3
Shear injury. The patient has a recent history of severe trauma to the head. Axial FLAIR MRI (a), T2* GRE (b), DWI (c), and ADC map (d) show edema and hemorrhage within the corpus callosum with associated restricted diffusion in the splenium of the corpus callosum (arrows)
Traumatic brain injury and hemorrhage related to alcohol. Traumatic brain injury (TBI) is a serious public health problem in the United States. Each year, at least 1.7 million traumatic brain injuries occur and contribute to substantial morbidity and mortality. Preexisting alcohol abuse is common among persons who incur TBI, with as many as 58 % reporting a history of alcohol abuse or dependence and as much as 25 % reporting previous treatment for substance abuse in one study. In a separate study analyzing 2,657 trauma patients experiencing TBI, 58 % had a heavy drinking history, 46 % had a positive blood alcohol level at the time of injury, and 36 % were intoxicated. Alcohol intoxication resulted in a greater likelihood of intubation, intracranial pressure monitoring, respiratory distress, and pneumonia. Timely management of TBI can significantly alter the clinical course if detected with neuroimaging within 48 h of the injury. Clinical manifestations that suggest major injury such as worsening level of consciousness, loss of consciousness for more than 5 min, focal neurological findings, seizure, failure of the mental status to improve over time, signs of a basal or depressed skull fracture, or confusion on examination almost always merit imaging. On the other hand, even patients with absence of clinical findings and high-risk circumstances can have intracerebral hemorrhage on imaging. Neuroimaging also plays an important role in the diagnostic workup of TBI. MRI is superior to CT in detecting axonal injury and cerebral contusions. However, conventional CT generally remains the initial imaging modality of choice during the first 24 h following injury. Alcohol intoxication can lead to severe injuries related to high-speed motor vehicle collisions while driving intoxicated or falls related to drunkenness, resulting in skull fractures, intracranial hemorrhage, cerebral contusions, and shear injury (Figs. 2.3 and 2.4). Alcoholism may also predispose to large and recurrent intracranial hemorrhage with relatively minor trauma due to underlying coagulopathy related to hepatic dysfunction (Fig. 2.5). Alcohol abusers also have a tendency to engage in violent behavior when drunk and may present with maxillofacial fractures, for which CT may be obtained (Fig. 2.6). Incidentally, these individuals often have a high burden of dental disease that may also be encountered on imaging.
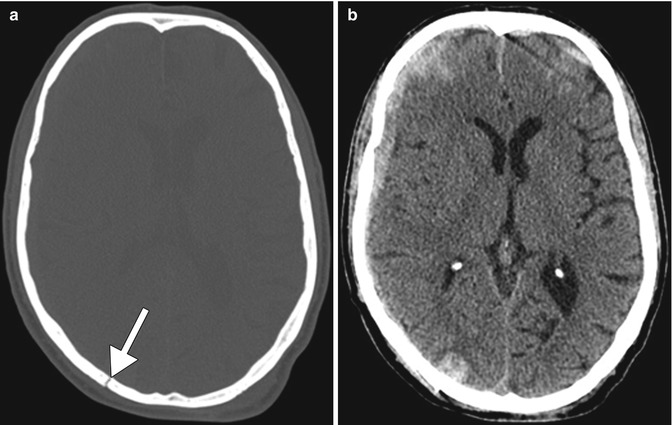
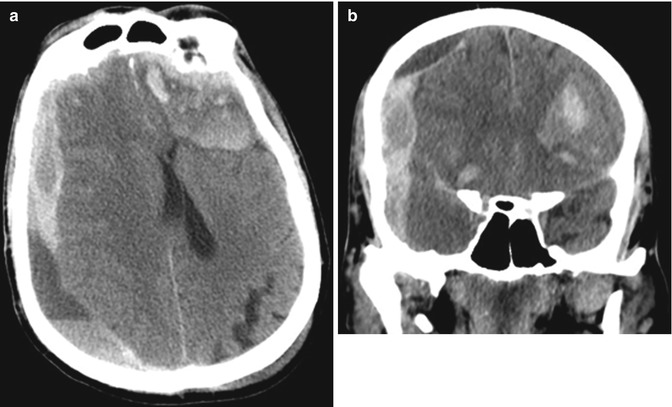
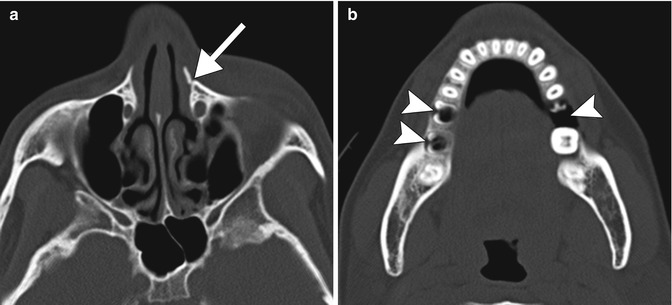

Fig. 2.4
Alcohol-related traumatic brain injury. Axial CT images in the bone (a) and brain (b) windows show a nondepressed right occipital bone fracture (arrow) and scattered acute intraparenchymal hemorrhage, including a right cerebral convexity subdural hematoma, subarachnoid hemorrhage, and hemorrhagic contusions in a patient who fell while drunk

Fig. 2.5
Acute upon chronic intracranial hemorrhage in a patient with alcohol-induced hepatic coagulopathy. Axial (a) and coronal (b) show a heterogeneous acute upon chronic right cerebral convexity subdural hematoma and a left frontal lobe hemorrhagic contusion and areas of subarachnoid hemorrhage in a patient with alcohol-induced liver failure and trauma

Fig. 2.6
Alcohol-related violence with maxillofacial trauma. Axial maxillofacial CT images (a, b) show a fracture of the left frontal process of the maxilla (arrow) and extensive dental disease (arrowheads) in a relatively young patient
Hepatic encephalopathy (also refer to Chaps. 28, 41, and 49 ). Hepatic encephalopathy (HE) may occur as an acute, potentially reversible disorder, or it may occur as a chronic, progressive disorder that is associated with chronic liver disease. Depending on the duration and extent of hepatic dysfunction, HE may be classified into fulminant hepatic failure (acute) or portosystemic encephalopathy (chronic). Clinical manifestations of HE can range from mild confusion to coma. Cerebral edema and raised intracranial pressure can result from fulminant hepatic failure and contribute to encephalopathy, but some degree of cerebral edema has been shown in patients with all grades of hepatic encephalopathy. Patients with the portosystemic encephalopathy frequently have pairs and triplets of abnormal astrocytes with a characteristic structure known as Alzheimer type II astrocytosis, in which the astrocytes exhibit physiological and functional abnormalities. When the liver is damaged, either acutely or over time, it can no longer remove neurotoxic substances such as ammonia and manganese from the blood. High levels of ammonia can have deleterious effects on neurotransmitter activity, impairment of cerebral metabolism, and altered neuronal gene expression. Manganese can deposit directly in the basal ganglia and induce extrapyramidal symptomatology. Manganese can also act synergistically with ammonia to activate peripheral-type benzodiazepine receptors and the gamma-aminobutyric acid (GABA)-ergic neuroinhibitory system. In acute HE, T2 and DWI abnormalities are present in the cerebral cortex, particularly in the frontal, temporal, and insular lobes, which correspond to edema (Fig. 2.7). MRS often demonstrates an elevated glutamine peak and diminished myoinositol and choline peaks. The differential diagnosis of acute hepatic encephalopathy includes other toxic-metabolic derangements, viral encephalitis, status epilepticus, and Creutzfeldt-Jakob disease. In chronic hepatic encephalopathy, there can be patchy high signal intensity on T1-weighted images in the bilateral globi pallidi, cerebral peduncles, and anterior pituitary (Fig. 2.8), secondary to manganese deposition. The differential diagnosis of hepatic encephalopathy includes total parenteral nutrition also secondary to excess manganese.
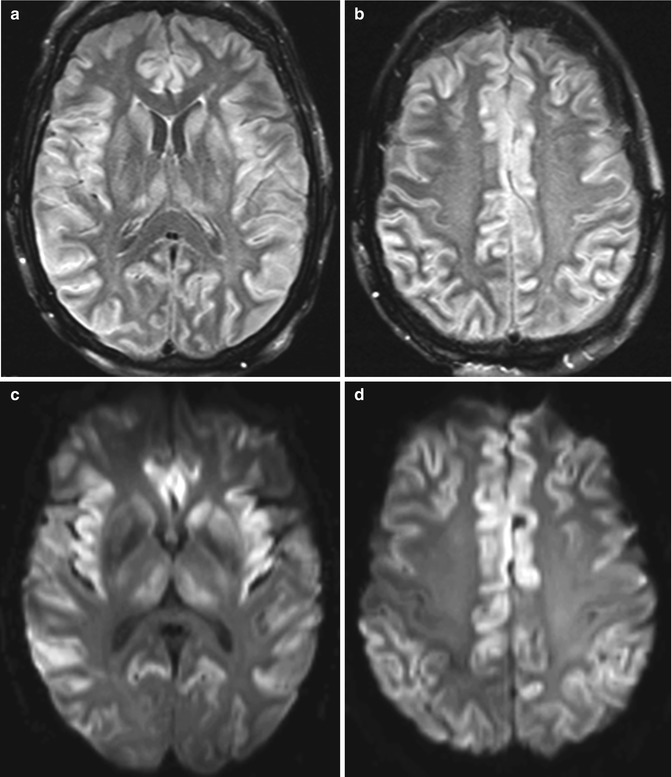
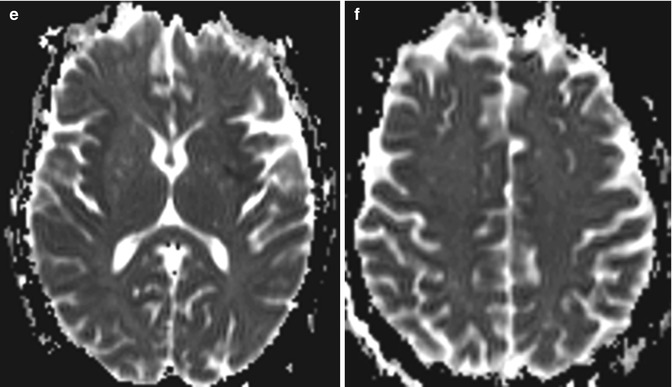
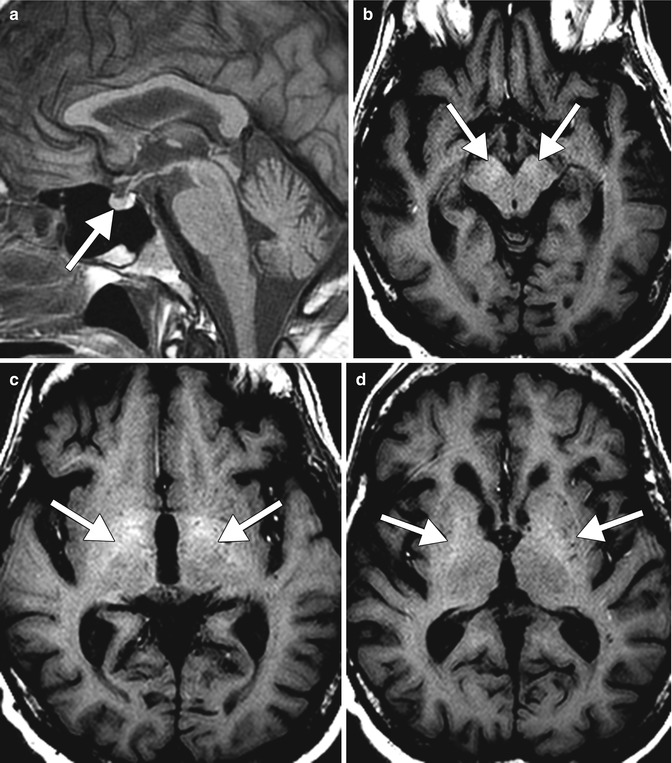


Fig. 2.7
Acute hepatic encephalopathy. Axial FLAIR MR images (a, b), DWI (c, d), and ADC maps (e, f) show diffuse gyriform hyperintensity and restricted diffusion predominantly affecting the bilateral frontal, insular, and temporal lobes

Fig. 2.8
Chronic hepatic encephalopathy. Sagittal T1 MRI (a) shows hyperintensity within the anterior pituitary (arrow). Axial T1 MR images (b–d) show hyperintensity within the bilateral basal ganglia and cerebral peduncles (arrows)
Osmotic demyelination (also refer to Chap. 35 ). Osmotic demyelination consists of vacuolization and intramyelinitic splitting with eventual rupture of the myelin sheaths and most commonly occurs in the setting of rapid correction of hyponatremia in patients with chronic alcohol abuse and malnutrition. The resultant pathologic changes include symmetric myelin disruption in areas with admixed gray and white matter, particularly the basis pontis and basal ganglia but also on occasion the thalamus, and neocortical and cerebellar gray-white junctions with corresponding T2 hyperintensity on MRI (Fig. 2.9). On DWI, corresponding restricted diffusion can be detected within 24 h after onset of symptoms and can serve as an early indication of this disease. The differential diagnosis for osmotic demyelination includes infarcts and microangiopathy, other types of demyelinating processes, neoplasms, and metabolic syndromes, such as Leigh disease and Wilson disease.
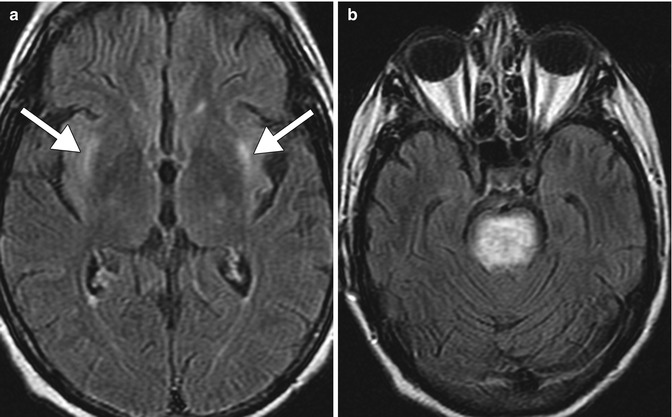

Fig. 2.9
Osmotic demyelination. Axial FLAIR MR images (a, b) show signal abnormality within the bilateral external capsules (arrows) and central pons
Wernicke encephalopathy. Wernicke encephalopathy is an acute neurological syndrome that results from thiamine (vitamin B1) deficiency, which can occur in alcoholics as well as nonalcoholics with nutritional deficiency. Thiamine deficiency impairs enzymes resulting in glutamate accumulation and cell damage with disruption of the cell membrane osmotic gradient, leading to edema. This condition was initially described by Carl Wernicke in 1881 as “superior acute hemorrhagic poliencephalitis.” The classic triad of ataxia, oculomotor abnormalities, and confusion is present in only 16–38 % of patients with Wernicke encephalopathy. Consequently, the new diagnostic criteria include 2 out of 4 of the following findings: oculomotor abnormalities, cerebellar dysfunction, and altered mental status or mild memory impairment. Characteristic imaging findings in alcoholic Wernicke encephalopathy include T2 hyperintensity and variable degrees of enhancement in the medial thalami, mammillary bodies, tectal plate, and periaqueductal gray matter (Fig. 2.10). Diffusion-weighted imaging findings in Wernicke encephalopathy ranges from decreased, normal, or increased ADC values. In contrast to alcoholic Wernicke encephalopathy, involvement of the cerebellum, cerebellar vermis, red nuclei, dentate nuclei, splenium of corpus callosum, fornix, cerebral cortex, cranial nerve nuclei, and basal ganglia has been identified only in nonalcoholic and pediatric patients with Wernicke encephalopathy (Fig. 2.11). The differential diagnosis of symmetric lesions of the medial thalami should include artery of Percheron infarct, deep cerebral vein thrombosis, virus encephalitis, acute disseminated encephalomyelitis, CNS lymphoma, and Creutzfeldt-Jakob disease variant (refer to Chap. 49).
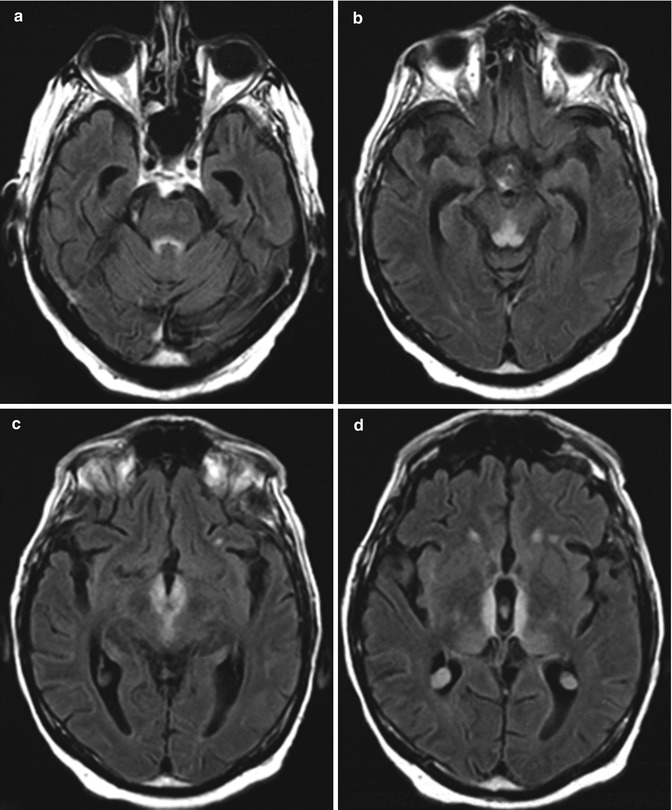
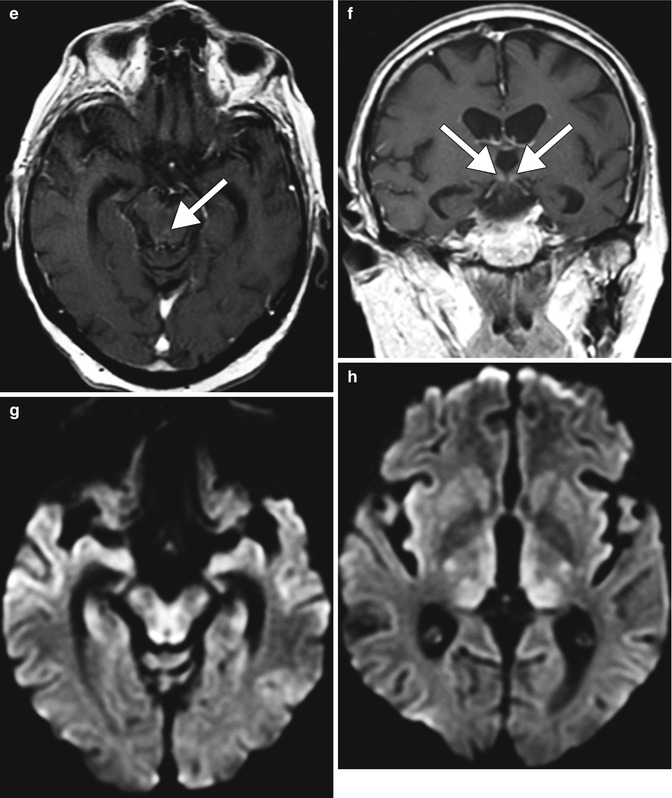
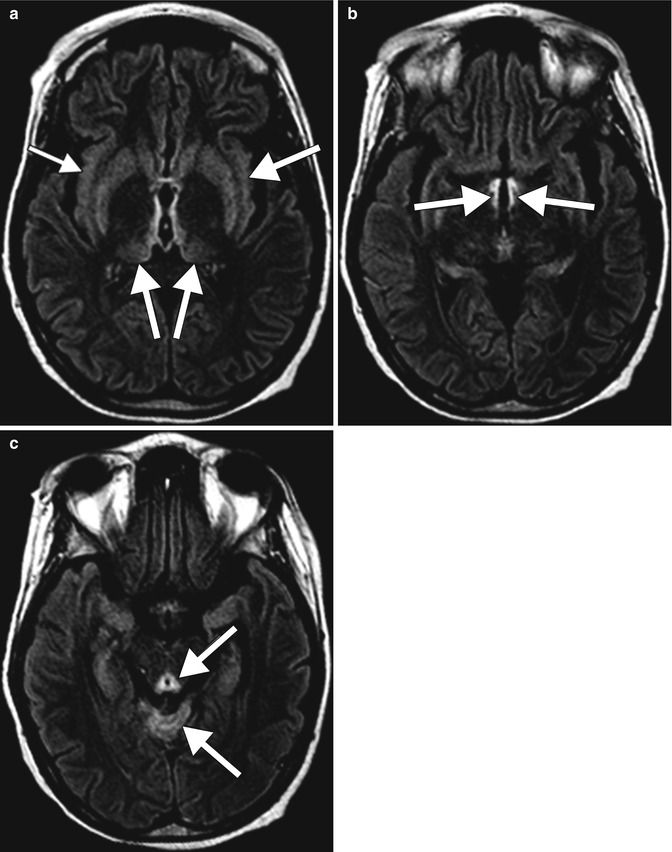


Fig. 2.10
Alcoholic Wernicke encephalopathy. The patient was found confused with whiskey bottles scattered throughout the home. Symmetric, abnormal T2 signal involving the periaqueductal gray, tectal plate, medial thalami, hypothalamus, and mammillary bodies with mild associated enhancement (arrows)

Fig. 2.11
Nonalcoholic Wernicke encephalopathy. Axial FLAIR MR images (a–c) show hyperintensity in the mammillary bodies, hypothalamus, thalamus, cerebellar vermis, and periaqueductal gray matter in a symmetric distribution (arrows)
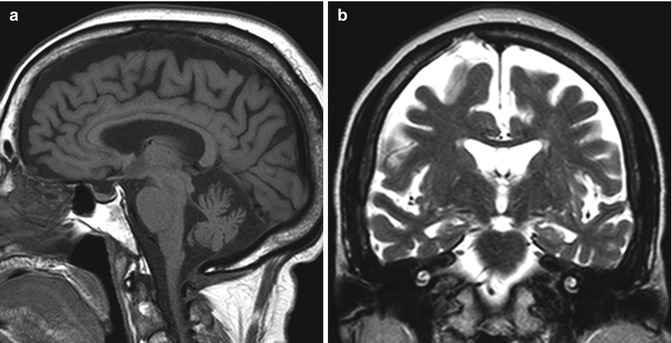
Cerebral atrophy. Several studies on alcoholics have demonstrated significant volume loss in cortical and subcortical brain regions. Although there can be diffuse cerebral volume loss with chronic alcoholism, the frontal lobes, hippocampi, and cerebellar vermis are disproportionately affected (Fig. 2.12). Since the frontal lobes help regulate judgment, risk taking, motivation, mood, and wanting, the degeneration that occurs here is contributory to continued drinking despite awareness of these negative consequences. In addition to morphologic volume loss and resultant ex vacuo ventricular dilatation that can be demonstrated on both CT and MRI, 1H MRS generally demonstrates lower concentrations of NAA and choline-containing compounds, most notably in the frontal lobes, medial temporal lobes, and cerebellum. Cross-sectional diffusion tensor imaging studies in alcohol-dependent individuals have indicated decreased fractional anisotropy and increased mean diffusivity in the genu, body and splenium of the corpus callosum, as well as in the centrum semiovale, which suggest compromised axonal/myelin integrity. Several other conditions can cause brain atrophy that resembles the effects of chronic alcoholism, including frontotemporal dementia and the effects of antiepileptic medications, such as dilantin (refer to Chap. 27).