(A) Expression of different glutamate receptor subtypes, demonstrating the complexity expected in any attempt to modulate glutamatergic neurotransmission. (B) Illustration of the corticostriatal circuits, demonstrating the role of glutamatergic neurotransmission in motor, limbic and associative loops. (C) An early schematization of the dopaminergic/glutamatergic imbalance in Parkinson’s disease and psychosis, as a tribute to the first pharmacologist who developed amantadine and memantine.
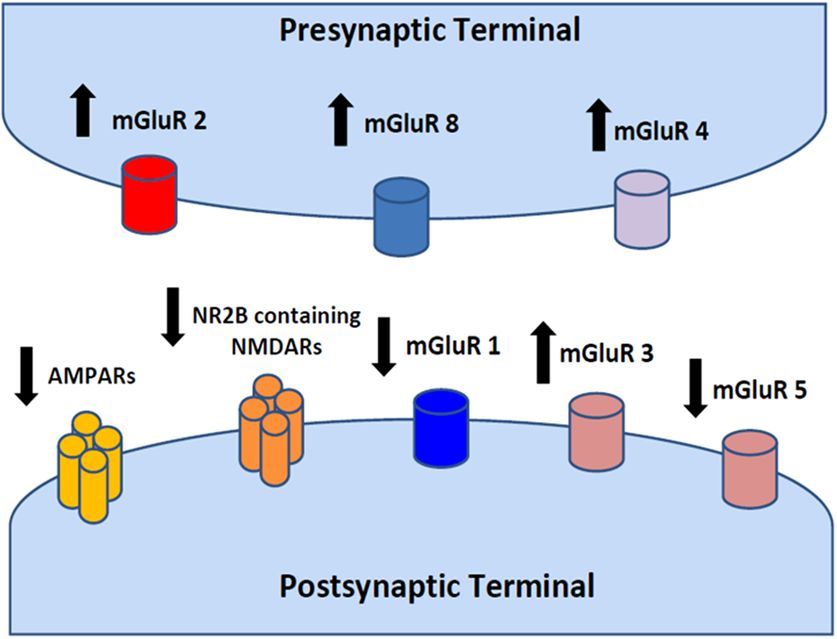
Schematic depiction of a glutamatergic synapse and localization of glutamate receptors. Arrows up or down indicate the possible receptor pharmacological modulation (activation vs inhibition). Glutamate receptors can be divided into two groups according to their molecular structure and pharmacodynamic mechanisms of activation. Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels activated by glutamate interaction with the receptor. Metabotropic glutamate receptors (mGluRs) indirectly modulate several neuronal targets (ion channels, transporters and intracellular enzymes) by the activation of intracellular signaling cascades. iGluRs have been classified, on the basis of their pharmacological profile, in AMPA, NMDA and kainate receptors. According to the more recent nomenclature, iGluR subunits can be classified as GluA1−4 (or GluR1−4) for AMPA receptors, GluK1−5 (or GluR5−7 and KA1 and KA2) for kainate receptors and GluN1−3 (or NR1−3) for NMDA receptors. mGluRs can be divided into three groups: group I (mGluR1 and 5) receptors are coupled to activation of phospholipase C; and group II (mGlu2 and −3) and group III (mGlu4, −6, −7 and −8) receptors are negatively coupled to adenylyl cyclase and inhibit cAMP formation.
Summary of glutamatergic agents acting on ionotropic (A) and metabotropic (B) receptors and the pharmacodynamic mechanisms of each drug
Evidence-based results and meta-analyses
Evidence-based evaluations, promoted by scientific societies and by the Cochrane networks, by analyzing studies performed with blinded procedures versus placebo, concluded that amantadine is efficacious in reducing dyskinesias in PD [19, 20]. This evidence-based conclusion stems from the evaluation of several studies showing that amantadine reduces dyskinesias when administered orally at doses of 100–300mg/day [24] or intravenously as single dose of 200mg [25]. This evidence-based analysis included studies performed from 1998 to 2010 [19]. Effects were obtained in parallel-group or crossover designs. The duration of the studies was 2–6 weeks. Among the different studies in evidence-based and Cochrane reviews, only two protocols had prolonged follow-up of patients. These studies showed an apparent contradiction: one showed that after 6–8 months dyskinesias reoccurred, suggesting that tachyphylaxis superseded [24]. The other study reached a different conclusion: by withdrawing amantadine in patients, they observed an increment of dyskinesias and conclude that this finding showed that amatadine has a persistent effect on dyskinesias [26]. However, the withdrawal effect and tachyphylaxis are due to different mechanisms and so should be considered separately for further discussion on other activities of the drug.
None of the other glutamatergic drugs received an evidence-based score for any effects. Evidence-based evaluation did not support the effect of amantadine on PD symptoms or progression.
A single meta-analysis [22] confirmed a statistically significant effect of amantadine in the reduction of dyskinesias, with a large effect size. This study also evaluated memantine, dextrometorphan and remacemide, and showed that these antiglutamatergic drugs are effective on dyskinesias, but the power and reliability reached by these other drugs were not statistically significant.
Minor studies
Dyskinesias
Beyond amantadine [27–32], reduction of dyskinesias has been described for dextromethorphan [11], remacemide [33] and memantine [13–17]. Dextromethorphan [11] administered alone at doses of 60–120mg/day was tested in 18 patients and in 12 more patients in association with quetiapine sulfate: this combination is currently available only in the USA, but the US Food and Drug Administration (FDA) indication is only for emotional incontinence of pseudobulbar symptoms. Remacemide reduced dyskinesias at a dose of 150–600mg/day in one study performed in 39 patients [33]. Memantine reduced dyskinesias in two patients as reported in two centers [13, 14], but an earlier study on 12 PD patients showed no effect [34].
Cognitive improvement
Amantadine has been used to improve cognition in patients affected by traumatic brain injury and minimally conscious state [35, 36]. A single case study described improvement following amantadine treatment in a patient in a vegetative state [37].
No evidence has been provided in PD, but in an observational study it was shown that amantadine reduced the incidence of dementia in a group of treated patients [38].
Memantine is used for the treatment of early- and late-stage of dementia. Three studies have described memantine effects in Lewy body dementia and PD with dementia [39–41].
Memantine improves the activities of daily living (ADL) and instrumental activities of daily living (IADL) but is limited by the same number needed to treat (NNT) ratio observed with cholinesterase inhibitors: one of seven treated patients [40] showed an appreciable improvement, i.e. the test score improvement was better than the single-item unit increment.
Akinetic crisis and neuroleptic malignant syndrome
Akinetic crisis, also termed malignant syndrome, parkinsonism–hyperpyrexia syndrome, neuroleptic-like malignant syndrome or acute akinesia, is a complication that appears in parkinsonism because of treatment manipulations or withdrawal, infectious disease, trauma or gastrointestinal tract disease [42, 43]. Akinetic crisis is the most severe complication of PD, occurring with an annual incidence of three cases per 1000 parkinsonian patients, and consist of worsening of acute motor symptoms characterized by an akinetic state with dysphagia, hyperthermia, an increase in serum creatine phosphokinase and myoglobin, dysautonomia, and transient unresponsiveness to the current antiparkinsonian treatment or to increase in dopaminergic doses [42]. Its symptoms are identical to the symptoms of neuroleptic malignant syndrome, and it was hypothesized that both represent idiosyncratic severe complications due to heterogeneous causes [44]. The guidelines of the German Neurologic Society suggest that amantadine sulfate should be used via intravenous injection at a dose of 200mg to treat neuroleptic malignant syndrome and akinetic crisis. However, no evidence for this conclusion has been provided [45].
Psychosis induction
Anecdotal reports described the incidence of psychosis and hallucination in patients treated with amantadine and memantine [46–54]. A risk of psychosis induction was reported in cautionary guides for the side effects of this drugs, and reviews of safety show that the incidence is in the 1–5% range [19]. We found six reports of psychosis induced by amantadine [46–51] and three reports of psychosis induced by memantine [52–54], both administered at the current therapeutic dosages. A single case report described a complex psychosis with release of the maniac state and multiple ICDs in a patient treated with amantadine after several years of bipolar disorder (treated with antipsychotic drugs) and with late occurrence of PD [51].
Impulse control disorders and other compulsive disorders
Memantine is considered a possible option for the treatment of psychiatric disorders other than dementia: gambling [55], compulsive buying, binge-eating disorders [56], refractory obsessive-compulsive disorder [57] and pediatric obsessive-compulsive disorders [58] were improved in patients treated with memantine, but these studies are anecdotal, open label or have been presented in pilot studies or review.
Acamprosate is used for the treatment of alcohol craving at doses of 1200–2000mg/day: for this compulsion, acamprosate is registered in Europe and other countries as its use has been supported by blinded studies [59].
There are no reports challenging the proposed effects of memantine or acamprosate on compulsive behaviors, while for amantadine, discussion of its possible effects on ICDs is more lively. A study designed as a double-blinded crossover with placebo and a washout period of the effect of 200mg amantadine on gambling behavior in PD patients showed that amantadine reduced gambling (measured as Yale–Brown obsessive-compulsive scale scores and daily expenditure in gambling) in 17 patients [60]. A follow-up of this study suggested that the effect of amantadine was mediated by reduced risk-seeking behaviors [61]. An earlier anecdotal report showed that amantadine reduced gambling and punding behaviors in a lady affected by PD [62].
One further single case report showed that amantadine reduced gambling in one patient [63], and an open-label study suggested that amantadine may reduce punding [64].
As soon as the original study on gambling in PD was presented, two reports took a stand against the assumption that amantadine may reduce gambling or ICDs [65, 66]. The first one was a retrospective cross-sectional study that analyzed data from a study on dopamine agonist administration performed in order to understand the impact of these drugs on gambling and on ICDs in PD [65]. Data from this study were reanalyzed and the population treated with amantadine was compared with the untreated population; the study concluded that gambling was more common in patients who received amantadine and other drugs than in patients not receiving amantadine. However, this study provided no information and consistency of use (doses and timing, early or late add-ons, drug treatment duration) or other confounding factors of amantadine administration. The statistical results were significant only for part of the population – patients from the USA – and were not significant for patients from Canada, indicating a location bias. Moreover, in this study, dyskinesias that are reduced by amantadine, as shown by evidence-based reports [19], were more frequent in the in amantadine group than in the untreated group. This incongruity showed the confounding characteristics of any retrospective analysis that is unable to define the specific timing of treatment.
The second observational retrospective study [66] was presented as a comment on the crossover study on amantadine. In this study, gambling was also more common in the patients taking amantadine than in patients not exposed to the drug. Again in this study, the consistency and duration of treatment were not described. Dyskinesias were more frequent in the amantadine-treated group, yet even more relevant flaws could be shown in the statistical comparison, as no differences emerged from the treated and untreated groups if the comparison was adjusted for age, gender and disease duration and was restricted (as demanded by proper statistical methods) to patients with ICD.
Beyond the specific comments on these confutational reports [67], the essential element arising from this discussion is that a classical statistical epidemiological concept was not considered in either study, i.e. observational or cross-sectional studies cannot lead to cause-and-effect conclusions on drug efficacy because consistency of use, treatment duration and confounding by indication bias cannot be analyzed [68].
The confounding report of cross-sectional or observational studies can be exemplified by the experience with levodopa [69]. After the initial report by Cotzias, the use of levodopa was challenged by reports that showed no efficacy or only side effects [69]. The key to efficacy was patient selection and dose finding. Only after the appropriate PD staging systems were developed and the action mechanisms clarified did levodopa became the mainstay in PD treatment. Thus, our conclusion is that appropriate studies on the effects of amantadine and other antiglutamatergic drugs on ICDs should be designed and performed not just to provide a single indication for use, but mostly to highlight new research pathways [70]. Among the different antiglutamatergic drugs discussed in the present chapter, amantadine has several flaws that impinge on its chance of being involved again in prospective or blinded studies. Among these flaws is the fact that amantadine is an old drug [18, 20] with no patents forecasting revenues from the study, its production is not expensive and thus returns and costs are not important, and economical support for further studies is almost hopeless. A further flaw is that amantadine is an apparently well-known and widely used drug, even though its real mechanisms of action are poorly understood and guidelines suggest that it could worsen cognitive functions; it is nevertheless used for improving the outcome of brain trauma. It has a low dopaminergic activity but reduces dyskinesias. Cochrane and evidence-based reviews and treatment guidelines report insufficient evidence and low recommendations for the use of amantadine in early PD as properly blinded studies have not been performed [19]. The only class I level A studies were relative to amantadine use in late PD with dyskinesias [19]. Thus, amantadine use should not be undertaken lightly in early PD, yet it is still largely used, as shown by retrospective studies [66]. Exposition to amantadine is burdened by tachyphylaxis [24], but this effect is poorly studied, and its presence would imply that any study will require, as a prerequisite, absence of prior exposure to the drug.
Conclusion
In this chapter, we have emphasized controversial results and debates rather than focusing on the well-known effects of amantadine and antiglutamatergic drugs, which are described in detail by several previous reviews and analyses [19–21]. These reports are succinctly summarized, as widely known or available. All previous reviews concur in showing four main effects for antiglutamatergic drugs:
Antiglutamatergic drugs may reduce dyskinesias, and the strongest statistical support of this effect has been produced for amantadine [19–22]. Other antiglutamatergic drugs may share the same effect, but none is supported by statistically sound studies.
Antiglutamatergic drugs may improve cognition, but only the effect of memantine is statistically supported [39–41].
Any antiglutamatergic drug may induce psychosis, and this effect may severely impinge our conclusion of studies inadequately supported by statistical analyses.
Antiglutamatergic drugs may reduce ICDs and other compulsive disorders, but only acamprosate is registered and only for the treatment of alcohol cravings [59].
The first consideration provided by our review is focused instead on the controversial results, including no effects of memantine on dyskinesias, no effect of amantadine on ICDs and the controversial effects on cognition.
Our consideration is that, for any further study, adequate methodological approaches should be devised: patient selection should consider prior exposure to antiglutamatergic drugs, as complex tachyphylaxis and withdrawal effects have been described [24, 26]. Doses, duration of treatment and timing (i.e. antiglutamatergic administration before or after dopaminergic treatment) could clarify the proper approach to this class of drugs.
As a concluding hypothesis, we would like to focus on the peculiar cognition improvement/ICD reduction/psychosis induction pattern that can be identified, at least, for amantadine and memantine. We suggest that this pattern is dependent on an inverted U-shaped curve [71] of responses to this class of drugs: antiglutamatergic drugs, as shown by the apparently contradictory findings, may present with positive or negative effects.
Inverted U-shaped curves can be defined as a Yerkes–Dodson-type inverted U-shaped function [71], which represents a system that reaches its optimal function when the midrange level of a variable is reached, while both higher and lower levels are associated with impaired functions. Figure 3.3 shows examples of inverted U-shaped curves devised to explain the effects of dopaminergic drugs on behavior or on activity of the dorsal or ventral striatum. In the figure, we also propose an inverted U-shaped curve depicting the effect of antiglutamatergic drugs. In this case, mild levels of glutamatergic blockade may improve cognition by inhibiting the corticostriatothalamic loop (Figure 3.1) and by reducing recurrent behavior patterns. However, further blockade may excessively inhibit the cortical projections and release-stereotyped behaviors, interfering with cognition.
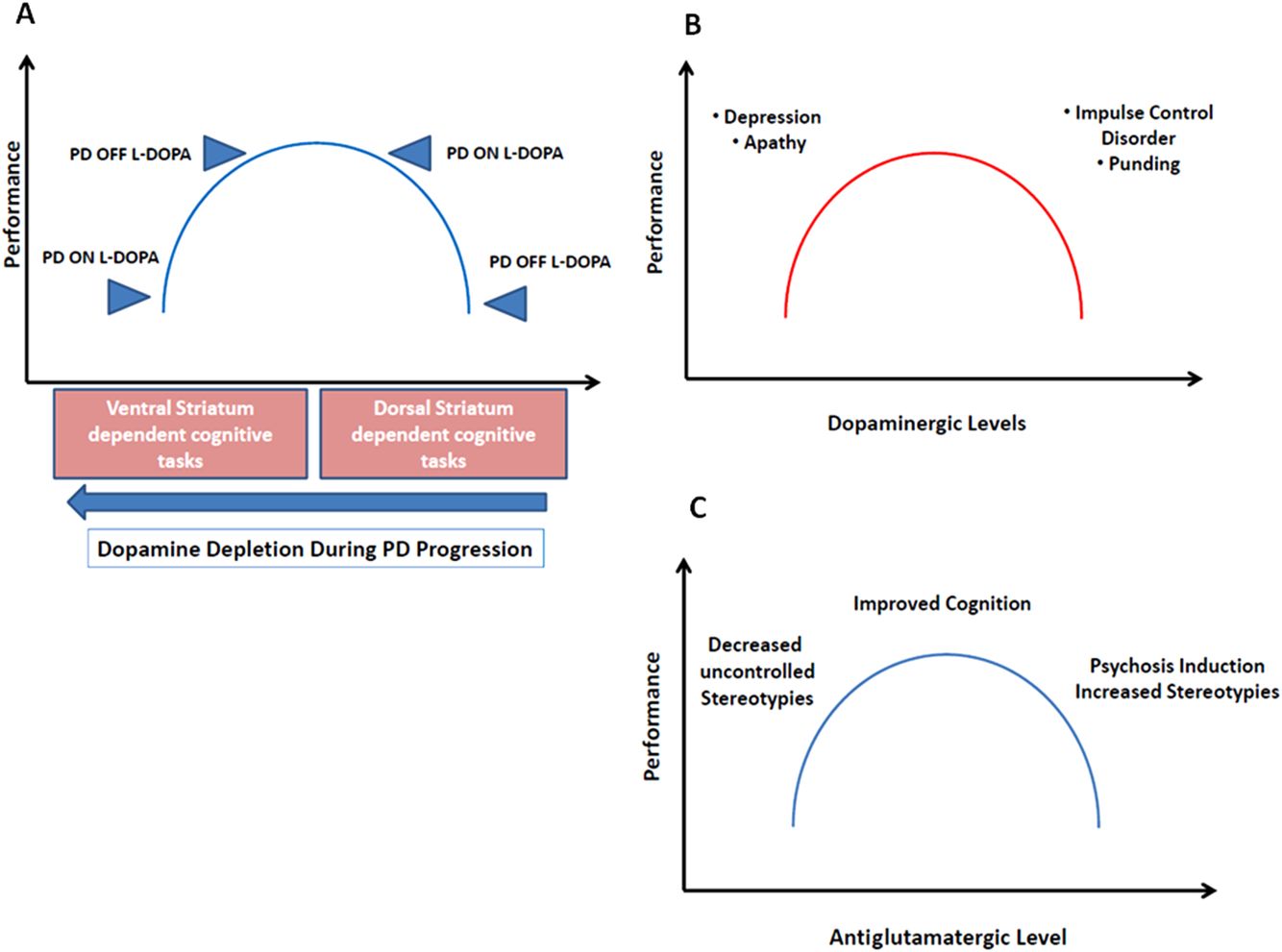
Examples of inverted U-shaped curves, focused on dopaminergic effects (A, B) and antiglutamatergic effects (C).
As described in the introduction, glutamatergic modulation is perhaps more complex than dopaminergic modulation: for the latter, several phenomena had to be identified and studied, such as priming [74] and the effects of continuous versus pulsatile administration [75], in order to understand the mechanisms underlying erroneous predictions or results.
The clinical phenomenology of PD has also changed in recent years, from the plain motor phenotype to the complexities of mental disorders that were denied initially [76] and later became a core element in PD evaluation, to the point of classifying PD as a “neuropsychiatric disorder” [77]. In a temporal gradient, hallucinations and then depression and dementia, followed by ICDs and then functional overlays and somatoform disorders were identified [78]. In parallel, the idiopathic nature of PD was challenged by genetic evidence, to the point of proposing the concept of Parkinson’s syndrome rather than PD [77].
We believe that understanding the effects of antiglutamatergic drugs in parkinsonism will require the same amount of conceptual and research efforts that were devoted to dopaminergic drugs.
The take-home message is that amantadine may be used for transient dyskinesia reduction, once other management options have been considered, as tachyphylaxis or complex effects of amantadine may play a role.
Memantine may be used for cognition improvement with the caveat that confusion or psychosis might worsen, rather than improve. However, the results of blinded studies on large patient populations suggest that the risk of these side effects is modest [39–41].
The presence of ICDs would suggest the use of antiglutamatergic drugs only as a speculative option. Add-ons of amantadine, memantine or acamprosate may reduce some compulsive behaviors, but our practical suggestion is that this approach should be used in a temporarily restricted frame, in order to slowly taper off dopamine agonist drugs and postpone/avoid the occurrence of the dopamine agonist withdrawal syndrome [80]. Clinical acumen will be needed to discern which patients are suitable for this mostly off-label trial, or for the use of antipsychotic drugs or for accelerated tapering of dopaminergic treatment. Confidence should come from properly designed studies on new drugs, and from characterization of responders and nonresponders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



