Fig. 1
Brain water content at 24 h after surgery. Brain water content was increased in the right frontal lobe at 24 h after SBI. This increase was significantly reduced with both high-dose and low-dose EAA therapy: sham group (79.71 ± 0.38 %); SBI group (82.76 ± 1.21 %); low-dose EAA (79.83 ± 1.12 %); high-dose EAA (450 mg/kg) (79.71 ± 0.38 %). # p < 0.05 compared with vehicle group
EAA Therapy Improved Neurobehavioral Function Following SBI in Rats
The modified Garcia test was performed to evaluate sensorimotor deficits at 24 h post SBI. Vehicle-treated SBI rats (14.50 ± 1.23) showed significant neurobehavioral deficit compared with the sham group (19.83 ± 0.88; p < 0.05). Low-dose EAA (150 mg/kg) rats showed a significant improvement in neurobehavioral function at 24 h (18.33 ± 0.85) compared with the vehicle-treated SBI group (14.50 ± 1.23; p < 0.05). High-dose EAA (450 mg/kg) rats showed a significant improvement in neurobehavioral function (18.50 ± 0.76) compared with the vehicle-treated SBI group (14.50 ± 1.23; p < 0.05) (Fig. 2).
Discussion
Neurosurgical procedures can lead to postoperative cerebral damage at the margins of the operative site. This SBI can damage viable brain tissue unintentionally by a wide range of methods used during neurosurgical interventions. The concern with these injuries is the heightened inflammatory response that can propagate direct cell death that, in turn, can enhance BBB disruption, with subsequent increase in brain edema and deterioration in neurobehavioral function. Even if patients survive the insult, currently there are limited effective treatment options to help them recover afterwards. Similarly, effective pretreatment therapies to help prevent and/or reduce SBI damage are also limited. In the present study, we investigated the effects of EAA therapy on SBI, including the potential of this therapy to reduce brain edema and thus prevent the development of sensorimotor behavioral deficits.
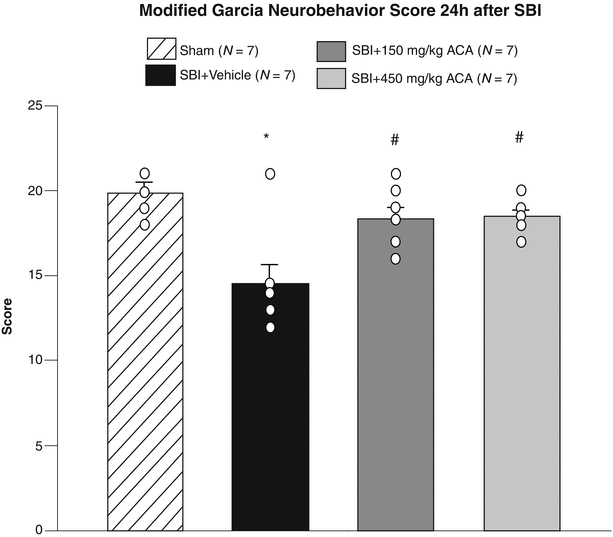

Fig. 2
Neurobehavioral function at 24 h after surgery. Vehicle rats had significant neurobehavioral deficits compared with sham rats. Both low- and high-dose EAA treatment significantly improved neurobehavioral testing scores at 24 h post SBI: sham group (19.83 ± 0.88); SBI group (14.50 ± 1.23); low-dose EAA (150 mg/kg) (18.33 ± 0.85); high-dose EAA (450 mg/kg) (18.50 ± 0.76). *p < 0.05 compared with sham group; # p < 0.05 compared with vehicle group
Structural brain damage manifesting as cell death and edema following SBI is not an uncommon phenomenon. Previous work in SBI has shown massive neuronal degeneration and death in the area of injury [12], along with findings of increased expression of proapoptotic factors, inflammatory mediators, and proteolytic enzymes throughout the brain and in circulation. Furthermore, BBB integrity can be compromised through matrix metalloproteinase upregulation [2, 9, 15], which leads to propagation of inflammatory cell-mediated cerebral damage and worsening of cerebral edema. One of the orchestrators of BBB disruption is plasmin.
EAA acid is a well-recognized lysine antifibrinolytic analogue. It works primarily by competitively inhibiting the conversion of plasminogen to plasmin in the bloodstream [3]. By doing so, EAA can reduce the levels of active plasmin and potentially preserve the neurovascular unit of the brain. Previous studies in the pediatric extracorporeal membrane oxygenation patient population found that EAA significantly reduced the incidence of intracerebral hemorrhage through BBB preservation, possibly through plasmin inhibition. In the spontaneous intracerebral hemorrhagic stroke population, research suggests that EAA could decrease hematoma sizes in stroke victims by reducing plasmin-mediated neuronal damage [10]. Other studies showed that re-hemorrhage following subarachnoid hemorrhage was significantly reduced, in part because of plasmin inhibition [5, 11]. These studies suggest a role for EAA therapy to block the effects of plasmin on BBB disruption and, in doing so, improve the neurological outcome of subject populations. We found that EAA therapy before surgery resulted in reduced cerebral edema and with the apparent structural protection, neurobehavioral function was well preserved. This is similar to previous work, suggesting that EAA may provide a therapeutic regimen necessary to block plasmin-mediated neurovascular and neurologic damage. Our study had limitations in that we did not study the potential mechanism of EAA-induced reduction in brain edema following SBI. Further studies are warranted to determine how EAA mediates preservation of the neurovascular unit following SBI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








