Algorithm for the diagnosis of mild cognitive impairment.
To illustrate diagnosis according to Figure 15.1: an initial clinical and cognitive evaluation may show that the patient has experienced an objectively confirmed decline in memory functioning but activities of daily living (ADLs) are largely preserved. The patient can be diagnosed as aMCI. Continuing down the diagnostic algorithm, if memory alone is impaired then the patient is diagnosed with aMCI single domain. If other domains, typically language, executive function or visuospatial skills, are impaired then the patient is diagnosed as having aMCI multiple domain. Similarly, the right-hand arm of the algorithm would be followed if the patient showed cognitive decline but no memory impairment. In the same way, impairment would be characterized as affecting single or multiple domains. Much subsequent research has shown that aMCI patients have a greater risk of developing AD dementia than individuals with no cognitive impairment. The most recent diagnostic guidelines, published in 2011 by an international working group [15], suggested new criteria for “MCI due to AD”. This term is synonymous with aMCI, and indeed the core clinical features are almost identical to those proposed by Petersen and colleagues in their original exposition [12]. The new criteria serve two main purposes: first, to identify patients in the symptomatic predementia phase of AD and, second, to make a distinction between core clinical criteria based on cognitive and clinical symptoms, and research criteria incorporating the use of biomarkers.
Core clinical diagnostic criteria for MCI
A diagnosis of aMCI [15] is reached by evaluation of the following features: (Table 15.1).
Core clinical and cognitive criteria
(i) Complaint of change in cognition reported by patient or an informant, or assessed by clinician based on previous cognitive assessment
(ii) Objective evidence of impairment for age and education, in one or more cognitive domains, and typically including memory
(iii) Preserved independence in activities of daily living
(iv) Not demented
Additional criteria for MCI consistent with AD
(i) No evidence of vascular, traumatic, or other medical causes of cognitive decline
(ii) Evidence of longitudinal decline in cognition
(iii) Where relevant, history consistent with AD genetic factors
Table 15.2 provides descriptions of three patients in order to illustrate key features of aMCI and the main differential diagnoses.
Case 1: Amnestic mild cognitive impairment
Mr S is a 69-year-old retired teacher of English. He gave a clear account of progressive symptoms he has been experiencing over the past 2 years. He said that he often goes to the fridge and forgets why, has difficulty recalling the names of people, and more recently has struggled to find words in conversation. He also finds that when dancing, an activity he has enjoyed for many years, he cannot recall the steps. His friend and dancing partner has commented upon this. He has lost confidence and is at times tearful, although this is not pervasive. More than 20 years ago, he suffered with depression as a result of his wife’s death. He lives alone, managing all domestic chores, although comments that he is “letting this go a bit.”
Key features:
Progressive cognitive symptoms.
Subjective memory complaints made by the patient and corroborated by a friend, and pertaining to a routine activity.
Suggestion of difficulties, but not a complete decline, with household chores that were previously managed independently.
Case 2: Alzheimer’s dementia
Mrs T is a 64-year-old woman with a 12-month history of progressive memory problems. She forgets recent events and conversations and has taken to making copious lists to which she continuously refers. Her husband reports that she is disorientated even in local shops that she has shopped in for many years. Mrs T is relatively unconcerned about these problems, although concedes that her memory is not as good as it was before. At interview, she could not recall what she had had for a meal the previous evening and was unaware, despite gentle prompting, that it had been her birthday the previous weekend. Her mood is reasonable and there is no history of anxiety or depression. Her husband says she was previously very organized and efficient, but that this is no longer the case. He said that he would not be happy to leave her alone for a weekend and would be worried about her ability to cook and look after herself.
Key features:
Progressive episodic memory problems.
Some lack of insight into the extent of cognitive problems.
Difficulties with activities of daily living.
Case 3: Worried well
Mrs B is a 53-year-old left-handed woman. She attended the appointment alone. At interview, she gives a detailed account of her circumstances and describes a 3- to 4-year history of problems with her memory. These problems are more noticeable in her workplace. She works in a very demanding role as a solicitor, and finds it increasingly difficult to remember what has been said in meetings. Although she is aware of these problems, there are no objective concerns from her employers and she has been performing to expectations. Mrs B denied any problems with activities of daily living and continues to cook and shop for her family. Her mood is good, but she described her sleep as being poor in recent weeks.
Key features:
Able to give a full and detailed account of her history independently.
No evidence of progressive worsening of cognitive problems.
Able to hold down a demanding occupational role.
No disruption to activities of daily living.
No evidence of mood disorder.
Evidence of a change in cognition
The first line of inquiry should seek evidence of a change from the patient’s previous level of cognitive functioning. Such evidence may be provided by the patient, by an informant or by both. The diagnostic utility of subjective memory complaints alone is debated. Some studies have found subjective complaints to be useful for prediction of cognitive decline [16–19], and to be significantly associated with verbal memory performance [20], while others have reported no such findings. Busse et al. [21, 22] showed that the exclusion of subjective memory complaints increased the accuracy of dementia detection. Ahmed et al. [23] showed that MCI patients endorsed questions pertaining to episodic memory impairment in everyday scenarios, but that these complaints were not significantly different from complaints made by the “worried well,” i.e., patients with subjective but no objective memory impairment. A critical methodological issue is that the techniques used to assess subjective memory complaints vary widely across studies; some studies have used a single invalidated general question, while others have employed questions about everyday abilities or memory questionnaires. Memory as an overall function may be perceived as unimpaired and may not lead to a complaint, whereas questioning about memory in specific activities might reveal more pertinent information.
Objective cognitive impairment in one or more cognitive domains
The second criterion requires the presence of objective cognitive impairment, adjusted for age and education. In patients with aMCI, or “MCI due to AD,” episodic memory impairment is the salient feature, and may be isolated or be accompanied by less prominent impairment in other cognitive domains. There is no consensus as to which test or which cut-off score should be used to define “impairment” of memory. Screening tests (including instruments such as the Mini Mental State Examination, the Montreal Cognitive Assessment or the Addenbrooke’s Cognitive Examination) or more detailed neuropsychological evaluation can aid in documenting cognitive function. Typically, impairment is defined as a score falling more than 1.5 standard deviations below age appropriate normative scores [11]. Petersen [14] recommended that the criterion for objective memory impairment should be taken in conjunction with subjective experience of a change in cognition, as the latter identifies decline in comparison to previous levels of functioning, and objective memory corroborates the complaint.
Preserved independence in functional abilities
The third criterion requires intact ADLs such as cooking, washing, and personal hygiene. While people with MCI may take more time over these activities and make occasional errors, their general independence should be maintained. This criterion is assessed, for the most part, by obtaining a history from the patient and an informant. Scales for the measurement of ADLs are also available but are not in widespread usage (e.g., [24]). It is recognized that application of this criterion is challenging as it requires knowledge about the individual’s expected level of function in their usual environment at the current stage of their life. It may also be difficult to judge between decline in physical functioning that is age related or related to coexisting medical problems, and that which is related to cognitive impairment. Given that any degree of cognitive impairment will have an effect on certain activities, Perneczky et al. [25] differentiated between complex ADLs, where even mild degrees of cognitive impairment affected function, and basic ADLs, which were unimpaired in MCI. Basic ADLs refer to day-to-day core abilities, such as eating, washing, walking, and dressing. Complex or instrumental ADLs are defined by their higher level of complexity, incorporating tasks such as managing finances and medication and meal preparation [26]. Key to MCI diagnosis is that functional independence is preserved, since impaired ADLs constitute a cornerstone of dementia diagnostic criteria.
No dementia
The final criterion requires that the person is not demented, judged on the basis of the previous three criteria, and on the clinician’s judgement. As discussed, a core reference is criterion three where the degree of functional impairment can determine diagnosis of MCI or AD dementia. This requirement is understood to mean that the patient does not meet diagnostic criteria for dementia at presentation, and not that there is no underlying pathological change [27].
Epidemiology
Annual conversion rates
A large body of research has shown that if the core clinical criteria outlined by Albert et al. [15] are positive for a diagnosis of MCI, there is a greatly increased risk of developing dementia. The annual rate of “conversion” to dementia ranges from 6 to 25% across studies [28, 29]. Researchers at the Mayo Alzheimer’s Disease Research Center followed a group of 220 individuals who met criteria for MCI as originally defined [30], for 3–6 years. In this study, approximately 12% of patients progressed to dementia per year, a figure some ten times the expected population incidence for dementia of 1–2% per year. After 6 years of follow up, approximately 80% of this MCI group had progressed to dementia. Further longitudinal studies have demonstrated a high conversion rate from aMCI to AD dementia specifically [31, 32], although a wide discrepancy in the progression rates is evident (6 to 46%). This variability may be due to the source of the subjects (e.g., ascertainment bias in highly specialized research clinics), research methods, implementation of criteria, and length of follow-up. Nevertheless, it has been argued that, given a long enough follow up period, most and possibly all MCI will progress to dementia [33], Studies with follow-up exceeding 5 years reveal that patients continue to convert to dementia even after long periods of time. By 8–10 years, some 50–80% of MCI patients will have converted [29, 33].
Prevalence
Estimates of the prevalence of MCI in the community have largely been based on Petersen’s [14] criteria. The Mayo Clinic Study of Ageing documented an overall prevalence of MCI in the community at 16%, and this prevalence increased with age, positive APOE4 status, and was higher in men and unmarried subjects. Prevalence decreased with higher levels of education [34]. More specifically, aMCI was 2.3 times more common than naMCI. A study from Leipzig, Germany yielded an overall prevalence of 19.2% in subjects aged 75 years and older, but found naMCI to be as frequent as aMCI [35]. Despite differences in methodology, sample sizes. and population demographics, most studies have reported prevalence figures for MCI to be in the range of 11 to 20%.
Cognitive phenotype of aMCI
With the drive towards aMCI as a therapeutic target, understanding the nature of cognitive impairment is imperative in order to inform the development of tools for early and accurate diagnosis. The necessity is clearly illustrated by studies showing that minor differences in the definition of aMCI can result in up to a three-fold difference in the prevalence rate [36, 37].
Studies examining the cognitive features of MCI have consistently found that episodic memory impairment is the salient initial impairment, and is a predictor for progression to dementia, irrespective of the sample selection or setting [5, 9, 38–40].
Episodic memory impairment
A series of key publications has revealed that aMCI patients suffer from impairments in the encoding and storage of new information, and are insensitive to retrieval cues or structure inherent in the learned material. The impairment has therefore been interpreted as one of new learning, rather than either the accelerated long-term forgetting seen in patients with epilepsy [41], or the disrupted retrieval that is seen in some patients with frontal brain lesions [42].
While episodic memory impairment is largely accepted to be the earliest manifestation, there is less consensus regarding the specific memory processes impaired, and no recommendation for any particular memory test to be used. Examination of episodic memory in aMCI has traditionally used word list learning or story recall tasks, the majority of which require free recall of stimuli after a delay. Alladi et al. [43] examined the diagnosis of aMCI depending on the memory test used. In a sample of 124 non-demented patients, 58% fulfilled criteria for aMCI based on a verbal list learning memory task (the Rey Auditory Verbal Learning Task [RAVLT]; 73% fulfilled criteria based on a verbal and/or a visual task (the RAVLT and Paired Associate Learning task [PAL] [44]). If impairment on both a verbal and visual task was required, prevalence fell to 41%. Verbal memory is typically used to make a diagnosis but if used alone, a sizeable number of cases may not be detected.
More recent work has suggested that the assessment of long-term memory consolidation may be a particularly sensitive diagnostic tool, particularly where impairments are subtle or masked by high premorbid levels of functioning. Walsh et al. [45] controlled for differences in initial learning of a story-learning task in aMCI patients. Recall was tested at a standard 30-minute delay and an additional 1-week delay. They found that aMCI patients showed an increased rate of forgetting illustrated by greater decline in recall compared to controls after 1 week. The authors propose that long-term memory consolidation is impaired in aMCI and that probing memory after a typical 30-minute delay may fail to detect relevant impairments.
A number of studies have also consistently noted impairment in the ability to learn new associations between stimuli in aMCI. Associative learning poses greater demands on cognitive processing because of the need to bind distinct elements. Hippocampal engagement has been shown specifically where associative processing is required [38, 46]. In particular, the Paired Associate Learning (PAL) test from the Cambridge Neuropsychological Test Automated Battery (CANTAB) has been shown to be a sensitive tool in aMCI. This computerized test uses delayed response to test the ability to associate two elements, an abstract visual pattern with a spatial location. The task employs purely visual stimuli rather than word based stimuli. This may confer diagnostic specificity since verbal material is more vulnerable to the effects of depression, a common differential diagnosis in memory clinic settings [47]. The task has been shown to be a sensitive discriminator between aMCI patients who will progress to AD compared to those who do not [44, 47], citing sensitivity and specificity indices in excess of 90% accuracy. Ahmed et al. [48] showed that the PAL is also sensitive to time to disease progression, suggesting a role in the evaluation of drug therapy interventions.
Impairment in other cognitive domains
Beyond the salient amnestic impairment, many aMCI patients have deficits in other cognitive domains when sensitive neuropsychological tests are employed.
Semantic memory
Testing of memory in a clinical setting is often confined to episodic memory, but there is accruing evidence that semantic memory is also impaired in aMCI. Semantic memory is memory for general concepts and facts that are not specific to one situation, and can be likened to the database that gives meaning to perceptual experiences. Studies in early AD have shown that the semantic memory deficit is attributable to deterioration in organizational structure [49]. A number of studies have reported consistent impairment for individual items across tasks, suggesting impairment in semantic memory and not a failure to access information [50–52]. Studies have shown the most sensitive tasks are those testing naming and generation of lists according to category. Knowledge for famous people also appears to be particularly vulnerable [53, 54], and the deficit extends to other proper name based information. Ahmed et al. [55] found that 87% of aMCI patients were impaired on at least one task of naming objects, famous faces or famous buildings. Semantic memory impairment may be a particularly useful marker of MCI due to AD dementia, given that normal elderly may show deficits on episodic memory tasks, but do not typically show semantic impairment [56].
Attention and executive dysfunction
Executive function refers to the cognitive process of planning, initiation, and regulation of behavior. These higher-order capacities encompass processes such as abstract thinking, initiation of appropriate action, and inhibition of inappropriate action and goal-directed behavior. The aMCI patients have been shown to be impaired on dual-task performance and planning and problem solving tasks (see [57] for a review). Impairment in inhibition relative to healthy controls has been demonstrated on the Stroop test [58] that requires suppression of non-congruent color naming. Tests of attention and memory in combination may also increase the accuracy of prediction of progression from aMCI to AD. Albert et al. [59] reported that a measure of memory and executive function was able to distinguish those MCI patients who progressed to AD from those who did not progress over 3 years, with an accuracy of 80%.
Language
A number of landmark studies have suggested that language abilities may be vulnerable very early in the Alzheimer’s disease process. The most commonly reported problems are anomia, diminished vocabulary, and word-finding difficulties, highlighting an early lexicosemantic processing deficit. In the Nun study – a longitudinal study of autobiographical data and diary entries collected from convent archives for 678 Catholic sisters – Snowdon et al. [60] showed that, in writings produced as early as the second decade of life, low idea density was associated with low cognitive performance in later life. Perhaps more remarkably the measure also emerged as a reliable predictor of AD at postmortem. Garrard et al. [61] showed that the last novel of Iris Murdoch, written a year before she was diagnosed with AD in her mid-seventies, contained a more restricted, and higher frequency vocabulary than her earlier works, again pointing to a lexicosemantic processing deficit. More recently, Ahmed et al. [62] conducted an individual case analysis of language impairment in an autopsy-confirmed case series of aMCI patients. Examination of linguistic assessments conducted from aMCI to severe-stage AD revealed a significant linear decline in syntactic complexity, and semantic and lexical content. The findings suggest that there is a progressive disruption in language integrity, detectable from the prodromal stage in a subset of patients with AD.
Visuospatial function
Parietal lobe dysfunction, related to visuospatial and visuoconstructional impairment, has long been recognized to be an early feature of AD [63, 64] and a significant predictor of patients with a rapid rate of disease progression [65]. Visuospatial abilities are commonly tested using block construction tests and tasks requiring the reproduction of geometric figures such as the Rey Osterreith Complex Figure (ROCF) [66] and the Clock Drawing Test (CDT) [67]. There has been considerably less research examining these deficits in MCI. De Jager et al. [68] used a modified version of the clock drawing test (CLOX) [69] and found that MCI patients were significantly impaired compared to controls, and that performance on this task could reliably discriminate between participants with MCI and AD. Similarly, Thomann et al. [70] showed significant stepwise impairment in clock drawing between healthy controls, MCI, and AD patients whereby all three patient groups significantly differed from each other. It should be noted, of course, that tests such as the CDT, which assess visuospatial abilities, rarely depend on a single cognitive domain, but require the integrity of a number of cognitive abilities [71].
Predictors of progression
A key clinical goal when faced with a patient with MCI is to be able to predict the likelihood and rate of future cognitive decline.
Many factors have been identified that correlate with prognosis in MCI, ranging across cognitive, imaging, cerebrospinal fluid (CSF), blood-based, and genetic markers. Some of these markers aim at disease specificity (e.g., detection of beta–amyloid in AD) whereas others measure downstream effects of disease (e.g., brain atrophy). There is as yet no consensus over the predictive capacity of individual or combinations of biomarkers, and many are not yet available in routine clinical practice. Nevertheless, a requirement for biomarker evidence of AD pathology has become widespread in recent clinical trials and other research environments.
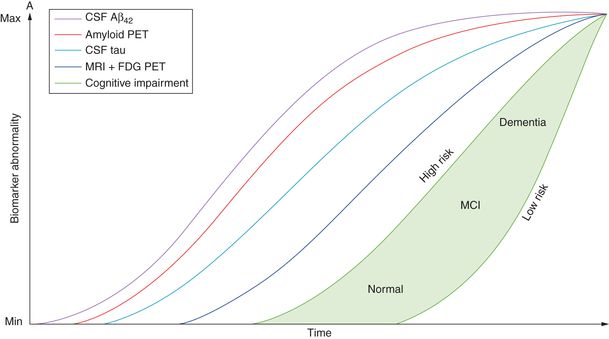
Indeed, recent diagnostic criteria reflect a reconceptualization of AD as a pathologic process beginning years, even decades, before the clinical onset of symptoms [15, 72]. Under this conceptualization, biomarkers will vary in sensitivity and specificity at different stages of the disease [72, 73] (Figure 15.2).
Neuropsychology
The more severe the memory impairment, the more likely and rapid will be the progression to dementia. Moreover, individuals with multidomain aMCI will progress more rapidly and die sooner than those with single-domain aMCI [74].
Brain imaging
Structural imaging
There is a large body of data showing that structural MRI can be used to predict the progression of MCI patients to dementia (see [75, 76] for recent reviews). Numerous studies have demonstrated reduced medial temporal lobe (hippocampal and entorhinal) volume in patients destined to convert from MCI to probable AD up to 2 years prior to clinical conversion, relative to MCI patients who remain stable [76–79]. Whole brain and ventricular volume [80], as well as the volume or cortical thickness of posterior regions including the posterior cingulate and precuneus [81, 82] have also been linked to progression from MCI to dementia.
Functional imaging
FDG-PET studies of patients with MCI have shown significant differences in resting cerebral glucose metabolism between MCI patients and healthy controls. These differences are most marked in the temporoparietal cortex, posterior cingulate, and temporal lobes, including the hippocampus [83, 84]. Temporoparietal and posterior cingulate hypometabolism has been shown to predict MCI conversion with an accuracy of between 75 and 95% [85–87]. However, FDG-PET is not universally available and its use in clinical practice remains generally restricted to large clinical centers.
Amyloid imaging
In recent years, a number of PET ligands have been developed that bind to amyloid plaques in the brain. Research has shown that imaging using these ligands reliably correlates with amyloid burden at postmortem examination [88]. Roughly 30% of cognitively normal individuals, 60% of MCI patients, and 90% of clinically diagnosed AD patients are amyloid positive on PET imaging [89]. MCI patients show greater tracer binding in the medial and lateral parietal lobe, anterior and posterior cingulate cortex, medial and lateral frontal lobe, and the lateral temporal lobe when compared with healthy controls [90, 91]. Conversion rates from MCI to dementia have repeatedly been shown to be increased in patients with positive or high retention on amyloid imaging [72, 92, 93]. Amyloid-positive individuals are also more likely to have abnormal CSF biomarkers [94] and show increased rates of brain atrophy [95] than amyloid-negative individuals. However, amyloid imaging is still only sparsely available and is expensive.
PET ligands that bind to hyperphosphorylated tau are currently being developed and evaluated in human subjects [96, 97].
CSF/blood biomarkers
CSF biomarkers of Alzheimer’s pathology include the total amount of tau (t-tau), which reflects the level of neuroaxonal degeneration, phosphorylated tau (p-tau), which correlates with tangle pathology, and the 42 amino acid isoform of amyloid β (Aβ42), which correlates inversely with plaque pathology.
A low level of CSF Aβ42 is thought to be the earliest currently detectable indicator of AD pathology [89]. A study by Buchhave et al. [98] found CSF Aβ42 levels to be abnormal in MCI patients 5–10 years before conversion to dementia.
Increased CSF total tau (t-tau) and phosphorylated tau (p-tau) levels are indicators of neural degeneration and correlate with structural MRI measures in AD, such as hippocampal volume [81]. CSF tau levels probably become abnormal later in the course of disease than Aβ42 levels, but track cognitive impairment more closely [89].
Studies examining the clinical utility of CSF biomarkers in predicting conversion from MCI to dementia have found that a combination of tau and Aβ42 yields a sensitivity of 80–90% and a specificity of 70–80% for predicting conversion to probable AD [99, 100]. The predictive accuracy varies across studies due to differences not only in the patient populations and follow-up intervals, but also in the methods for specifying biomarker cut-offs. Standardization of methods for CSF biomarker measurement will be critical if their use is to become widespread in clinical settings [101].
CSF examination is invasive so the identification of blood-based AD biomarkers would be very helpful for clinical practice. A number of recent studies suggest that such markers exist (e.g., [102–105]) but replication of these results in large, prospective cohorts is needed.
Apolipoprotein ε genotype
It is well established that carrier status for the ε4 allele of the ApoE gene is a risk factor for the development of Alzheimer’s disease, while the ε2 allele conveys a degree of protection from the disease [106]. The ε4 allele has been shown to predict progression from MCI to dementia in several studies, and in a meta-analysis [107]. Its presence has also been shown to determine the speed of hippocampal atrophy in cognitively normal adults [108]. Nevertheless, routine use of ApoE genotyping in clinical practice is not recommended [109].
In summary, patients with MCI show signs of AD-related pathology including brain atrophy, reduced glucose metabolism, amyloid accumulation, and abnormal levels of CSF Aβ42 and tau. These biomarkers often correlate with each other and predict future conversion from MCI to dementia. Biomarkers are now often employed in clinical trials and other research settings, and their use may soon be warranted more widely in clinical practice in order to personalize diagnosis and treatment as early as possible in the course of neurodegenerative disease.
Etiology and neuropathology
MCI can clearly be caused by a variety of conditions other than early AD pathology. The list of differential diagnoses is as long, or longer, than that for dementia and includes neurodegeneration (e.g., AD, frontotemporal dementia, dementia with Lewy bodies), vascular disease (e.g., vascular dementia), epilepsy (epileptic amnestic syndrome, transient epileptic amnesia), psychiatric conditions (e.g., depression, anxiety), and many systemic diseases (e.g., heart failure, diabetes mellitus, thyroid disease, systemic cancer). The clinical history, neuropsychological evaluation, laboratory tests, neuroimaging, and other investigations are all used to arrive at an etiological diagnosis, which can then guide treatment and help predict prognosis.
The neuropathological features of aMCI appear to be intermediate between those of normally aging controls and very early Alzheimer’s dementia [110, 111]. Neurofibrillary pathology in medial temporal lobe structures is thought to be the major substrate for memory decline [112]. Nevertheless, a range of associated pathological features is often also found, particularly cerebral infarcts and neocortical Lewy bodies [111]. These findings are in accordance with the observation that, while most people with aMCI will progress to AD dementia, some develop other clinical syndromes.
Management
Counseling
From a clinical perspective, it is important to emphasize to the patient and their family that aMCI is an abnormal condition and carries a risk of progressive memory impairment. Given a patient meeting diagnostic criteria for aMCI, and whose symptoms are felt to be caused by a degenerative process, it is reasonable for the clinician to counsel a 10–15% per year risk of progression to dementia. That risk will, however, vary according to the available evidence from clinical investigations. For example, marked hippocampal atrophy or clearly reduced CSF Aβ levels make the risk of progression greater.
Pharmacologic intervention
There are currently no Food and Drug Administration (FDA)-approved drugs for aMCI. Systematic reviews of randomized controlled trials of all cholinesterase inhibitors [113], donepezil [114], and galantamine [115] in MCI have found no evidence to recommend their use, and some claim that marginal beneficial effects are outweighed by the risks of adverse events. Studies of rofecoxib, Gingko biloba, vitamin E, and a range of other therapeutic agents have also failed to demonstrate any significant delay in progression to dementia (see [116] for review). These disappointing results reflect practical difficulties in studying MCI including heterogeneity in clinical populations, variability in outcome measures and the necessity for trials with a long duration. It is hoped that the use of biomarkers to enrich study cohorts with patients at higher risk for developing dementia will improve the sensitivity of clinical trials.
Non-pharmacologic intervention
Many physicians recommend lifestyle modification to try and slow the rate of progression. Physical exercise has well-documented benefits for general health and wellbeing, and epidemiological evidence links exercise with cognitive benefits and reduced risk of dementia. In patients with MCI, a few relatively small trials (reviewed in [117]) have shown modest benefits on certain specific cognitive measures, but these data are inconsistent and fail to show an effect on the most important clinical outcome – progression to dementia. Other work suggests that remaining socially active may protect against dementia [118]. While high-quality clinical trials in this area are lacking, lifestyle modifications such as these are unlikely to be harmful and may well have an overall benefit on quality of life in a proportion of patients.
Future directions: pre-MCI
While there is a correlation between AD pathology and observable cognitive impairment, it is also increasingly clear that the clinical syndrome becomes apparent long after the onset of pathological changes in the brain ([72]; see Figure 15.2). Even in subjects who show no evidence of dementia in life, postmortem analysis reveals AD pathology (Braak stage V and VI) in approximately one-third of the group [119, 120]. Recent literature has introduced the label “pre-MCI” to describe subjects who show some features of MCI on clinical examination, but no significant impairment on formal neuropsychological assessment, either with significant subjective memory complaints or biomarkers suggestive of AD (for example characteristic structural or functional brain imaging signature or elevated hyperphosphorylated tau or decreased Aß in the cerebrospinal fluid). Duara et al. [121] followed a group of pre-MCI subjects for 2–3 years. Over this time, 28% of pre-MCI subjects progressed to MCI or dementia, compared to only 5% in a healthy control group. Tests of memory, executive function, and ADLs were significant predictors of conversion. Storandt et al. [122] studied a group of 388 individuals diagnosed as CDR 0.5. Thirty-two participants met original criteria for aMCI [11], 90 met revised criteria for aMCI that allow for non-amnestic deficits [14], and 276 did not have cognitive impairment sufficient to merit diagnosis and were thus classed as pre-MCI. These groups were followed over 4 years where the outcome measure was progression to CDR 1, equivalent to a diagnosis of AD. Over the 4 years, the aMCI and revised aMCI group showed a conversion rate to AD dementia of about 12–15% per year, in accordance with previously cited rates [11]. The pre-MCI group also showed progression to AD dementia but at a slower rate with a mean survival of 8 years, given their subtle cognitive impairment at baseline. Of those cases which came to autopsy, neuropathological investigation confirmed that nine of nine aMCI, 18 of 20 revised aMCI, and 43 of 47 pre-MCI patients had AD pathology. The latter findings echo other autopsy analyses, which have shown that aMCI patients have a similar pathological load to AD, and therefore even aMCI may be considered a relatively advanced stage of disease.
In the long-awaited age of disease modifying therapies, pre-MCI is likely to be the best stage for intervention, but its accurate detection will require ongoing refinement of neuropsychological paradigms alongside the development of more sensitive and specific biomarkers for AD pathology.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree