Anatomy
The Cerebellum and Connections
I. External Architecture
Three Lobes—rostral to caudal
Anterior Lobe—functionally known as paleocerebellum or spinocerebellum
Posterior Lobe—functionally known as neocerebellum or pontocerebellum or cerebrocerebellum
Flocculonodular Lobe—functionally known as archicerebellum or vestibulocerebellum
Ten Lobules—names need not be learned, but tonsils are clinically important (i.e., tonsilar herniation)
Two Fissures
Primary—separates anterior and posterior lobes
Posterolateral—separates posterior and flocculonodular lobes
Peduncles—connect cerebellum with brainstem
Superior Cerebellar Peduncle (brachium conjunctivum)—connects the cerebellum to midbrain
Middle Cerebellar Peduncle (brachium pontis)—connects the cerebellum to the pons
Inferior Cerebellar Peduncle (corpus restiform or restiform body)—connects the cerebellum with the medulla and is the largest peduncle
The vermis (“worm”) lies between the two cerebellar hemispheres and is almost circular, its continuity interrupted in ventral sagittal plane only by the fourth ventricle.
The lateral recesses of the fourth ventricle communicate with the subarachnoid space via the foramina of Luschka.
II. Internal Architecture
Nuclei—each hemisphere contains four. The first three are in the roof of the fourth ventricle. These include:
Fastigial Nucleus—phylogenetically the oldest. Associated with the archicerebellum (vestibulocerebellum). Receives afferents from flocculonodular lobe and vermis; sends efferents direct via inferior cerebellar peduncle to vestibular nuclei (some fibers cross to contralateral cerebellum, loop around contralateral superior cerebellar peduncle [SCP], and reach vestibular nuclei and reticular formation via the uncinate bundle of Russell). This nucleus controls antigravity and other muscle synergies in standing and walking (proximal muscles).
Globose and Emboliform Nuclei (together known as the Interposed Nucleus)—lie slightly lateral to fastigial nucleus; receive afferents from paravermian region of paleocerebellum
or spinocerebellum; send efferents via SCP to contralateral red nucleus; concerned mainly with modulation of stretch reflexes (distal muscles).
Dentate Nucleus—largest nucleus. Located deep within white matter of cerebellar hemispheres; receives afferents from Purkinje cells of entire neocerebellum and part of paleo-cerebellum; also afferents from premotor and supplementary motor cortices via pontocerebellar system; efferents course through SCP, cross to opposite side at ponto mesencephalic border, and terminate in contralateral red nucleus and ventrolateral thalamus. Helps to initiate and control volitional movements (planning of movements).
Vestibular Nuclei—think of these as “displaced” cerebellar nuclei because of association with vestibulocerebellum. Function is to maintain gaze fixation and upright posture.
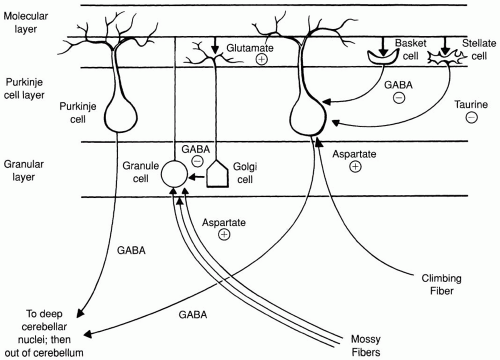
Microscopic Properties—(Figure 2-1)
Three cellular layers in the cortex (from outside to inside):
Molecular Layer—containing primarily basket cells, stellate cells, Purkinje cell dendrites, parallel fibers of the granule cells, and dendrites of the Golgi cells
Purkinje Cell Layer—contains only Purkinje cells
Granular Cell Layer—contains granule cells, Golgi cells, and glomeruli (bulbous expansions of mossy fibers where synaptic contact with granule and Golgi cells is made). This layer contains more neurons than the whole cerebral cortex!
Five cell types in the cortex:
Stellate and Basket Cells—local inhibitory interneurons that are excited by granule cells and inhibit Purkinje cells. Basket cells use γ—aminobutyric acid (GABA), and stellate cells are thought to use taurine as their neurotransmitters.
Purkinje Cells—large neurons that are the only cells in the cerebellum capable of transmitting efferent impulses from the cortex; also the only cells projecting out of the cerebellar cortex. These cells use GABA as their neurotransmitter.
Granule Cells—axons project upward to molecular layer as parallel fibers (run only parallel to longitudinal axis of each folia). The neurotransmitter is glutamate.
Golgi Cells—are located in the granular layer and send dendrites to the molecule layer. They are excited by granular cells and inhibit granule cells. They also are thought to use GABA as their neurotransmitter. They suppress the excitation of granule cells to mossy fiber input and curtail the duration of excitation reaching Purkinje cells.
Four afferent fiber types in the cortex:
Three bring information from outside the cerebellum:
Mossy fibers (make up 99% of incoming fibers)—transmit impulses exclusively from spinal cord and the vestibular and pontine nuclei by using granule cells as mediators; enter via all three peduncles. Thought to use aspartate as a neurotransmitter.
Climbing fibers—transmit impulses from inferior olives directly to dendrites of Purkinje cells of the opposite hemisphere. They are thought to use aspartate as a neurotransmitter. Stronger excitatory input than mossy fibers, and can enhance or inhibit mossy fiber input, thus functioning in motor learning.
Aminergic afferents—arise in Raphe nuclei and locus ceruleus. Raphe inputs are seratonergic and terminate in granular and molecular layers. Locus ceruleus inputs are noradrenergic and terminate in all three layers. Both inputs have widespread modulatory actions.
One fiber type—parallel fibers (axons of granule cells) originates within the cerebellum.
Note: Each Purkinje cell receives indirect input from >200,000 mossy fibers and only one climbing fiber. Both fiber types are excitatory.
Note: When a group of parallel fibers excites a row of Purkinje cells and neighboring basket cells, these basket cells inhibit distant Purkinje cells outside the band of excitation.
Contents of the Peduncles
Inferior Cerebellar Peduncle
Contains the following afferents:
Fibers from vestibular nerve and nucleus, terminating at the flocculonodular lobe (relayed to fastigial nucleus)
The olivo cerebellar tract, originating in contralateral inferior olive and terminating as climbing fibers directly on Purkinje cell dendrites
Posterior or dorsal spinocerebellar tract, which originates in Clarke column. Impulses transmitted here originate mainly in muscle spindles and are carried to the paravermian zone of the anterior and posterior lobes of the cerebellum (fastest conducting fibers in nervous system)
Fibers originating from accessory cuneate nucleus joining those of posterior spin-ocerebellar tract. Transmit impulses received by accessory cuneate nucleus from nuclei in middle and rostral portions of cervical cord above Clarke column (ascend in lateral cuneate fasciculus)
Fibers from brainstem reticular formation
Contains only one efferent, the fastigiobulbar or cerebellobulbar tract, representing the efferent limb of the vestibulocerebellar feedback circuit through which cerebellum influences spinal cord motor activity via vestibulospinal tract and medial longitudinal fasciculus.
Note: Inferior cerebellar peduncle contains all afferents, except the anterior spin-ocerebellar tract and the pontocerebellar fibers.
Middle of peduncle contains only afferents from the pontine nuclei (second neurons of fibers of the corticopontine tracts).
Superior cerebellar peduncle contains the following:
Only one afferent—the anterior or ventral spinocerebellar tract—which carries impulses from peripheral receptors (primarily Golgi tendon organs) to the paleocere-bellum (primarily vermis)
Numerous efferents (most of which eventually reach the cortex). Fibers originating in dentate and interposed nuclei that project through superior to contralateral red nucleus, ventrolateral and centromedian nuclei of thalamus, and brainstem reticular formation.
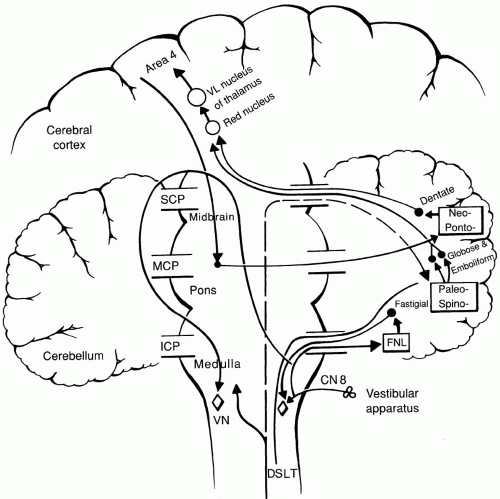
Note: Two important feedback loops exist, using the SCP for outflow:
Triangle of Guillain-Mollaret (Figure 2—2)—projections from red nucleus descend via the central tegmental tract to the ipsilateral inferior olive, from there to the contralateral cerebellar cortex (climbing fibers through inferior cerebellar peduncle), then to the dentate nucleus, then via the SCP to the contralateral red nucleus. With this circuit, the cerebellum indirectly modulates spinal cord motor activity by its connections with the red nucleus and reticular formation, from which the descending rubrospinal and reticulospinal tracts originate. The effect of cerebellar actions here is ipsilateral because of a double decussation (dentate to contralateral red nucleus; then rubrospinal fibers cross again in Forel decussation shortly after leaving red nucleus). Lesions anywhere along this circuit produce palatal mycoclonus, one of the few involuntary movements that does not go away during sleep (usually because of lesions of the central tegmental tract, which disinhibit the inferior olivary nucleus).
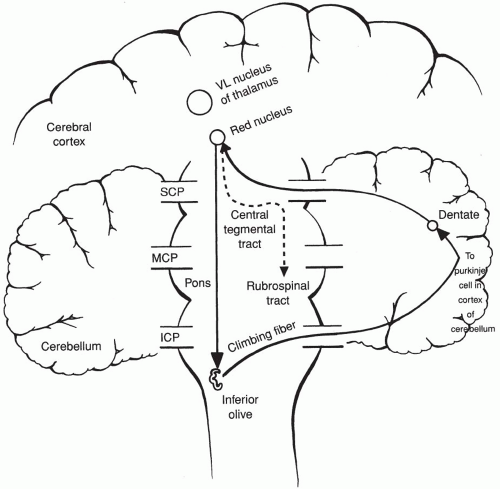
FIGURE 2-2. Triangle of Guillain Mollaret. VL, ventrolateral; SCP, superior cerebellar peduncle; MCP, middle cerebellar peduncle; ICP, inferior cerebellar peduncle.
Cerebellar feedback circuit via pontine nuclei—cerebral cortex to ipsilateral pontine nuclei (corticopontine fibers) to contralateral cerebellar cortex (mossy fibers via middle cerebellar peduncle) to dentate nucleus to contralateral ventrolateral subnucleus (VL) of thalamus to cortex.
III. Blood Supply
Superior Cerebellar Arteries—supply the bulk of cerebellum and peduncles; follow pontomesencephalic border, giving off branches to the tectum of the lower midbrain and superior cerebellar peduncles; these vessels run with peduncular fibers to deep nuclei (primarily dentate); supply ventral vermis and paravermis; continue on to supply rostral vermis and rostroventral portions of both hemispheres; give off small branches to almost every sulcus; cross tentorial margin on way to dorsal and lateral parts of rostral hemispheres.
Note: This feature makes these branches vulnerable to compression. Results in infarction of rostral cortex.
Anterior Inferior Cerebellar Artery—branch of basilar; supplies flocculus and adjacent convolutions.
Note: Internal auditory arteries supplying inner ear are usually branches of these.
Posterior Inferior Cerebellar Artery—branches of vertebral; some branches go to dorsolateral medulla (Wallenberg syndrome), caudal nuclei and inferior vermis, caudal cerebellum including tonsils
IV. Functional Considerations and Tracts (Figure 2-3)
General Information
Cerebellum acts as coordination center for maintenance of equilibrium and muscle tone via complex regulatory and feedback mechanisms. Also enables somatic motor system to accomplish discrete, skilled movements. It regulates movement and posture indirectly by adjusting the output of the major descending motor systems of the brain. It is a comparator, which compares intention with performance of movement and adjusts appropriately.
Vestibulocerebellum (Archicerebellum)
Input—vestibulocerebellar tract. Impulses from ipsilateral labyrinth travel via C8 to vestibular nuclei (some go directly as mossy fibers to cerebellar cortex); vestibulocerebellar tract fibers (mossy fibers) go from vestibular nucleus to flocculonodular lobe via inferior cerebellar peduncle to synapse on granule cells, which synapse on Purkinje cells in cortex of flocculonodular lobe. Also receives visual information from the lateral geniculate body, superior colliculus, and striate cortex, relayed via pontine nuclei.
Output—Purkinje cells in flocculonodular lobe send impulses to fastigial nucleus (this nucleus also receives inputs from vermis); from here fibers run via inferior cerebellar peduncle to ipsilateral lateral vestibular nucleus (fastigiobulbar or cerebellobulbar tract).
Some fibers cross to the other side of cerebellum, loop around contralateral SCP, and synapse on contralateral vestibular nucleus and reticular formation via the uncinate bundle of Russell. Fibers from vestibular nucleus and reticular formation form vestibulospinal and reticulospinal tracts, respectively, projecting to anterior horn cells.
Function—the maintenance of equilibrium, regardless of movement or body position, by synergistically modulating spinal motor impulses. Also help govern eye movements during stance and gait.
Lesions of the vestibulocerebellum cause the following:
Distorted equilibrium (on standing—astasia; on walking—abasia) with truncal ataxia and wide-based gait
Nystagmus
Tendency to fall toward side of lesion
Spinocerebellum (Paleocerebellum)
Input
Dorsal (posterior) spinocerebellar tract—impulses arising from Golgi tendon organs and muscle spindles travel via type Ia (FAST) fibers in posterior root and form
collaterals that synapse on second neurons in Clarke column (nucleus thoracicus) at medial base of dorsal horn from C8 to L2. Fibers from these neurons form dorsal (posterior) spinocerebellar tract and ascend ipsilaterally through inferior cerebellar peduncle to synapse on granule cells in the paravermian region (mossy fibers); these synapse on Purkinje cells.
Ventral (anterior) spinocerebellar tract (VSCT)—again, collaterals of Ia afferents synapse on neurons in medial portion of dorsal horns. These second neurons, found throughout the cord, send projections bilaterally, forming the VSCT, which passes through tegmentum of medulla, pons, and midbrain and reaches granule cells of spinocerebellum via the SCPs bilaterally; granule cells go to Purkinje cells. Signals in the VSCT are thought to reflect activity of segmental interneurons that integrate both descending and peripheral inputs. The VSCT neurons are principally driven by central commands that regulate the locomotor cycle. The VSCT input allows the cerebellum to monitor spinal circuit operation.
Note: This is the only afferent tract in the SCP.
Cuneocerebellar Tract—fibers from cervical dorsal roots (conveying position sense and deep sensibility of arms) ascend via cuneate fasciculus to accessory cuneate nucleus; from here, fibers travel with dorsal (posterior) spinocerebellar tract fibers to reach spinocerebellum via inferior cerebellar peduncle.
Note: These spinal impulses project to cerebellar cortex in a somatotopic pattern.
Inputs from auditory, visual, vestibular systems, as well as primary motor and somatosensory cortices have also been described.
Output—Purkinje cells of vermis project to fastigial nucleus, and those of para vermian region project to globose and emboliform nuclei. Efferents from fastigial nucleus project bilaterally to brainstem reticular formation and lateral vestibular nucleus. Additionally, crossed ascending projections reach the contralateral ventrolateral nucleus of the thalamus, and are relayed to the primary motor cortex (axial and proximal muscles). Efferents from the interposed nuclei cross in the decussation of the SCP to terminate on neurons of the magnocellular portion of the contralateral red nucleus. Some of these fibers ascend to the VL thalamic nucleus to synapse on neurons projecting to the limb areas of primary motor cortex. Fibers from the red nucleus cross back to form the descending rubrospinal tract, which modulates the activity of brainstem and spinal motor neurons.
Note: Test questions say only globose, emboliform, and dentate nuclei send projections through the SCP.
Note: There is a double decussation here—ipsilateral deep cerebellar nuclei go to contralateral red nucleus and thalamus/motor cortex; from there to crossing rubrospinal and corticospinal tracts to ipsilateral brainstem and spinal cord motor neurons.
Function—influences muscle tone, controlling collaboration between agonist and antagonist muscle groups; modulates activity of antigravity musculature, providing enough tone to maintain equilibrium while standing or walking. Controls the execution of movement.
Note: Spinocerebellum and vestibulocerebellum act together to control muscle tone and to ensure smooth, synergistic coordination of agonists/antagonists subserving gait and stance.
Lesions of the spinocerebellum cause the following (ipsilateral):
Truncal and limb ataxia
Proprioceptive errors of limbs
Gait disorders
Scanning speech (vermis)
Corticopontocerebellum (Neocerebellum)
Input
Corticopontocerebellar Tract—afferents from extensive areas of cerebral cortex, particularly areas 4 and 6, but also premotor and sensory cortices, form the corticopontine tract, passing through internal capsule and crus cerebri to synapse on ipsilateral pontine nuclei.
Note: Frontopontine fibers pass through anterior limb; all other fibers pass through posterior limb of internal capsule. Input crosses midline via pontine bridging fibers and enters contralateral cerebellar hemisphere via the middle cerebellar peduncle (pontocerebellar tract) to synapse on granule cells, to Purkinje cells.
Olivocerebellar Tract—fibers from inferior olive (climbing fibers) cross midline and enter the contralateral cerebellar hemisphere (posterior lobe) via the inferior cerebellar peduncle to synapse directly on Purkinje cell dendrites.
Note: Remember that inferior olives receive input from ipsilateral red nucleus via central tegmental tract (Triangle of Guillain-Mollaret).
Output
Dentatorubrothalamic Tract—Purkinje cells of entire neocerebellum and part of paleocerebellum project to dentate nucleus; from here, fibers project via SCP to contralateral red nucleus (parvocellular component) and ventrolateral thalamic nucleus (via the SCP decussation); from VL of thalamus, fibers pass through anterior limb of internal capsule to synapse in areas 4 and 6 of motor cortex.
Function—Neocerebellum receives information on each planned voluntary movement in advance and corrects by inhibiting some pyramidal and extrapyramidal motor impulses. It helps make complex voluntary movement smooth and precise.
Lesions of the corticoponto cerebellum cause the following:
Ataxia—particularly distal limbs, with deviation of gait and stance toward the lesion.
Dysmetria
Asynergia—decomposition of movement
Dysdiadochokinesia
Intention tremor—usually associated with dentate nucleus or SCP lesions
Rebound
Hypotonia (decreased deep tendon reflexes (DTR’s))
Delays in movement initiation
Inability to discriminate weight—objects feel lighter in the hand ipsilateral to lesion
V. Summary Table—Cerebellum and Connections
Archicerebellum | Paleocerebellum | Neocerebellum | |
|---|---|---|---|
Synonym | Vestibulocerebellum | Spinocerebellum | Cerebrocerebellum/pontocerebellum |
Lobe | Flocculonodular | Anterior | Posterior |
Nucleus | Fastigial/vestibular | Globose/emboliform | Dentate |
In-Peduncle | Inferior | Inferior/superior | Middle |
Out-Peduncle | Inferior | Superior | Superior |
Function | Equilibrium | Muscle tone | Compares planned to actual move |
Posture | Movement execution | Smooth precise movements | |
Eye movement | Stretch reflexes |
Thalamus
Function/Functional Subdivisions
Major relay and processing area for all sensory modalities (except olfaction) and motor input reaching cortex. Also important to autonomic function and arousal.
Specific Relay Nuclei—modality—specific (sensory and motor), topologically arranged, has discrete cortical projections (reciprocal). Examples: ventral posterior medial (VPM), ventral posterior lateral, internal capsule, ventral lateral (VL), ventral anterior (VA).
Nonspecific Nuclei—multimodal activation, widespread cortical projection, do not receive input from ascending tracts, involved with arousal. Examples: centromedian nucleus, parafasicular nucleus.
Association Nuclei—varied afferents/efferents to broad regions of association cortex. Examples: dorsal tier of lateral mass, anterior, dorsomedial.
Subcortical Nuclei—project mostly to other thalamic nuclei. Example: reticular nucleus. Anatomy: Dorsal portion of diencephalon (other parts are hypothalamus, subthalamus, epithalamus), divided by internal medullary laminae into medial and lateral masses, anterior nucleus, and intralaminar nuclei.
Medial Mass—only one major nucleus—dorsomedial.
Small—cell (parvocellular) component has reciprocal projection to prefrontal cortex, is involved with abstract thinking and long—term, goal—directed behavior.
Large-cell (magnocellular) part interrelates with hypothalamus, amygdala, frontal lobe, and is involved with olfactory activity.
Lateral Mass
Dorsal Tier—related to the association cortex, involved with integration of sensory information.
Lateral Dorsal: caudal extension of anterior thalamic nuclei, interconnects with cingulate gyrus
Lateral Posterior: interconnects with precuneus and receives input from superior colliculus.
Pulvinar: connects reciprocally with large association areas of parietal, temporal, occipital cortex. Also receives input from superior colliculus, reticular system, cerebellum.
Ventral Tier—posterior nuclei are concerned with all sensory modalities except olfaction; more anterior nuclei also have motor input and output.
Ventral anterior (VA): large-cell component receives input from substantia nigra. Small-cell portion receives input from globus pallidus. Output primarily to premotor cortex (area 6) for integration of movement.
Ventral lateral (VL): similar to VA with large- and small-cell components and similar input, but also has major cerebellar input and projects reciprocally with primary motor cortex (area 4).
Ventral posterior: main somatosensory and taste region, arranged somatotopically.
Ventral posterior medial (VPM): receives input from head through secondary trigeminal tracts and taste input from nucleus of solitary tract (located most medially). Projects to postcentral gyrus (areas 5,2,1).
Ventral posterior lateral: medial lemniscus system, spinothalamic tract from body input here. Lower dermatomes are located more laterally. Projects to cortex areas 3,2,1.
Metathalamic nuclei
Medial geniculate body: lies adjacent to superior colliculus, receives bilateral input from inferior colliculi, and projects to auditory cortex of superior temporal gyrus (Heschl, area 41).
Lateral geniculate body: six cell layers (three for each eye with 2,3,5 uncrossed); therefore, no cell in lateral geniculate body receives bipolar input. Projects to visual cortex (area 17) through retrolenticular limb of internal capsule and optic radiations (geniculocalcarine projections).
Anterior Thalamic Nucleus: actually a complex of three nuclei, input from mammilothalamic tract, with reciprocal output to cingulate gyrus, hypothalamus. Very important limbic system relay.
Intralaminar Nuclei: small collection of nerve cells, functionally the rostral extent of the ascending reticular system.
Centromedian Nucleus: input from globus pallidus and output to caudate and putamen.
Parafasicular Nucleus: input is from anterolateral system and cortical area 6, with output to caudate, putamen, and other thalamic nuclei.
Thalamic Reticular Nucleus: thin layer of cells between the posterior limb of internal capsule and external medullary laminae of thalamus. Does not project to cortex but does receive cortical input. Projects to other thalamic nuclei, brainstem reticular system, and itself. Nearly all thalamic efferents to cortex pass through here; therefore, may be important regulator of thalamic function.
Blood Supply
Posteromedial Arteries (Thalamoperforating): branches of posterior cerebral and posterior communicating arteries. Supply anterior and medial portions of thalamus.
Posterolateral Arteries (Thalamogeniculate): branches of posterior cerebral artery (PCA) that supply posterior thalamus (pulvinar) and lateral nuclei, including geniculate bodies.
Posterior Choroidal Artery: branch of the posterior cerebral artery that supplies choroid plexus of third ventricle and posterior thalamus.
Anterior Choroidal Artery: branch of the internal carotid artery that supplies subthalamus and ventral thalamus.
Clinical Correlates
Thalamic Aphasia: results from lesion of left thalamus, reflecting left hemisphere, producing deficits in language production, syntax, reception, or expression.
Nondominant Thalamic Lesions: may produce contralateral neglect and difficulties with spatial relationships.
Ventral Posterior Lesions: produce contralateral sensory loss to all modalities. Also may have thalamic pain syndrome in affected areas as “anesthesia dolorosa” in Dejerine-Roussy syndrome.
Lateral Geniculate Lesion: causes contralateral homonymous hemianopia. May accompany thalamic sensory or capsular stroke.
Bilateral Thalamic Lesions: most often vascular from anomalous blood supply; can impair consciousness and higher function.
Hypothalamus
I. Anatomy
The hypothalamus has direct connections with the posterior lobe of the pituitary (the neurohypophysis).
Supraoptic Nucleus—secretes vasopressin (antidiuretic hormone)
Paraventricular Nucleus—secretes oxytocin
Axons from the supraoptic and paraventricular nuclei project to the posterior lobe of the pituitary, and from here, the hormones (antidiuretic hormone and oxytocin, respectively) have direct access to the bloodstream.
Subnuclei and function
Suprachiasmatic: regulation of circadian rhythms
Anterior: temperature regulation
Ventromedial: satiety
Paraventricular: secretes oxytocin
Dorsomedial: rage
Posterior: heat conservation
II. Clinical Presentations
Diabetes Insipidus—can result from damage to osmoreceptors in the sulpraoptic nucleus
The hypothalamus regulates body temperature and food intake.
Stimulation of the lateral nuclei → hunger, gluttony
Stimulation of the ventromedial nuclei → satiety, decreased appetite
Limbic System
I. General Information
A collection of interconnected but not contiguous structures in the telencephalon.
Together with the hypothalamus and reticular formation, functions to maintain homeostasis, emotional and motivational states, arousal, memory, and learning.
The limbic pathways interrelate the telencephalon and diencephalon with medial midbrain structures
II. Components of the Limbic System
Hippocampal Formation
Dentate gyrus
Has a three-layered cortex: molecular layer, granule cell layer, polymorphic cell layer
Hippocampus (Ammon horn)
Also has a three-layered cortex: molecular layer, granule cell layer, polymorphic cell layer
Forms the floor of the temporal horn of the lateral ventricle
Covered by white matter called the alveus, which contains efferents from the hippocampus and subiculum, then forms the fimbria of the fornix
Fornix
The main efferent system for the hippocampus
Contains precommisural and postcommisural fibers (split by the anterior commisure)
precommisural fibers → septal area and anterior hypothalamus
postcommisural fibers → mammillary bodies
Subiculum
A zone of transition between the hippocampus and the parahippocampal gyrus
Parahippocampal gyrus
Part of the limbic system, but has a six-layered cortex like the neocortex
Contains the entorhinal cortex
Amygdala
Mammillary Bodies
Anterior Nucleus of Thalamus
Cingulate Gyrus
Entorhinal Cortex
III. Connections of the Limbic System
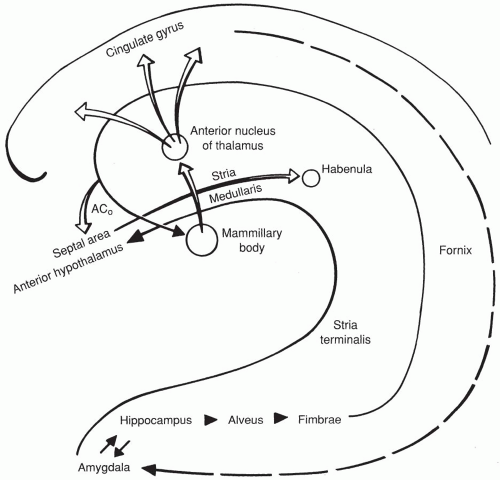
Circuit of Papez (Figure 2-4)
Hippocampus → alveus → fimbrae → fornix → mammillary bodies → anterior nucleus of thalamus → cingulate gyrus → amygdala → back to hippocampus
Stria Terminalis
From the amygdala → follows curvature of the tail of the caudate nucleus → to septal nuclei and anterior hypothalamus
Stria Medullaris
From septal nuclei and anterior hypothalamus → to habenular nucleus
Mammillotegmental Tract
Mammillary bodies → raphe nuclei of midbrain reticular formation
Medial Forebrain Bundle
Septal area and amygdala → raphe nuclei of midbrain reticular formation
IV. Clinical Correlates
Klüver-Bucy Syndrome—results from bilateral damage to the temporal lobes (such as herpes encephalitis, Pick disease). Classic symptoms: hyperoral, increased appetite, hypersexual, docile
Korsakoff Psychosis—may result from any disease of the temporal lobes. Common causes include thiamine deficiency (associated with Wernicke encephalopathy), third ventricular tumors, infarction (or resection) of inferomedial temporal lobes, and sequelae of herpes simplex virus encephalitis. Classic symptoms: amnesia (retentive memory impaired out of proportion to other cognitive functions), confabulation
Wernicke Encephalopathy—caused by thiamine deficiency; often seen in alcoholics. Pathologic changes occur in the mammillary bodies, dorsomedial nucleus of the thalamus, periaqueductal gray, and oculomotor nuclei. Classic symptoms: mental status change (delirium), ophthalmoparesis, ataxia, nystagmus. (mnemonic: MOAN)
Sham Rage—strong emotional outburst produced by stimulation of the amygdala
Hippocampal Sclerosis (mesial temporal sclerosis)—common cause for complex partial seizures (temporal lobe epilepsy)
Limbic Encephalitis—can be caused by herpes encephalitis or paraneoplastic disease
Transient Global Amnesia—loss of memory for minutes or hours without recollection of events during this period. Occurs in middle-aged or older individuals. Rarely recurs. Pathogenesis unknown; suggested causes include ischemia/transient ischemic attack, migraine, and complex partial seizure.
The Basal Ganglia
I. Function
To control and regulate the activities of the motor and premotor cortical areas via various reverberating circuits so that voluntary movements can be performed smoothly.
Motor activity is intricately controlled by the interactions of three major regions of the brain: cortex, cerebellum, and basal ganglia.
These three regions influence the lower motor neurons either:
Directly via the pyramidal system—the corticobulbar and corticospinal tracts
Indirectly via the extrapyramidal system—the basal ganglia
II. Anatomy
The basal ganglia consist of five subcortical nuclei:
Caudate—develops from the matrix around the lateral ventricle; derived from the telencephalon—part of the neostriatum
Putamen—developed and derived same as caudate
Globus pallidus—develops from the matrix around the lateral ventricle; derived from the diencephalon—part of the paleostriatum; divided into internal and external segments by the internal medullary lamina
Substantia nigra—also derived from the diencephalon
Subthalamic nucleus of Luys—also derived from the diencephalon
Note: The claustrum and amygdala were once considered a part of the basal ganglia, but they are now considered part of the limbic system.
III. Terminology
Striatum or Neostriatum or Striate Body = Caudate + Putamen
Corpus Striatum = Caudate + Putamen + Globus Pallidus
Pallidum or Paleostriatum = Globus Pallidus (internal and external segments)
Lentiform Nuclei = Globus Pallidus + Putamen
Nucleus Accumbens or Fundus of the Striate Body = where the head of the caudate and the putamen become one structure
IV. Functional Anatomy
The Caudate and Putamen
Develop from the same telencephalic structures and are composed of identical cell types. They are partially separated by the internal capsule, but connected by numerous gray bridges that cross through the internal capsule.
They can be thought of as one entity from a functional standpoint.
Caudate and putamen serve as the major input nuclei for the basal ganglia (i.e., they receive most of the input from the cerebral cortex).
The caudate—a large C-shaped mass of gray matter that is closely related to the lateral ventricle and lies lateral to the thalamus.
Composed of three parts:
Head—forms lateral wall of anterior horn of lateral ventricle
Body—long and narrow; forms part of wall of lateral ventricle
Tail—curves ventrally and follows the inferior horn of lateral ventricle into the temporal lobe, ends near the amygdala
The putamen—means “shell” in Latin (it covers the globus pallidus like a shell).
Bordered medially by the globus pallidus, laterally by the external capsule
Putamen + Globus Pallidus = Lentiform nuclei, but there is no functional overlap, this is an anatomical term only (because they lie like a lens-shaped wedge between the internal and external capsules). These two structures are very different in phylogenesis, structure, and function.
The Globus Pallidus
Resembles the substantia nigra pars reticulata histologically
The internal segment of the globus pallidus and the pars reticulata are strikingly similar in structure and function and can be considered a single structure divided by the internal capsule from a functional standpoint (much like the caudate and putamen).
Pars reticulata and the globus pallidus serve as the major output nuclei of the basal ganglia.
The Substantia Nigra
Means “black substance” in Latin
Lies in the midbrain and is composed of two zones:
Ventral (pale) zone—pars reticulata; resembles internal segment of globus pallidus; is GABA-ergic
Dorsal (dark) zone—pars compacta; comprised of dopaminergic neurons; has reciprocal connections with the striatum
The Subthalamic Nucleus
Involved in the indirect pathway
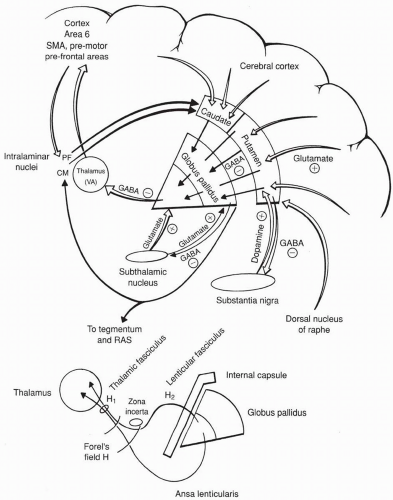
V. Basal Ganglia Connections (Figure 2-5)
The Direct Pathway
Input to the basal ganglia arises from the entire cortex and goes to the caudate/putamen.
Cortical projections to the striatum are topographically organized. Specific areas of the cortex project to different parts of the striatum and have specific functions.
Limbic lobes, orbitofrontal areas → nucleus accumbens and olfactory tubercle (ventral striatum) → ventral pallidum
Serves “limbic” functions such as motivation, aggression, sexual behavior, and some addictions
Area 8 (frontal eye fields), association areas → prefer caudate. Helps coordinate eye movements and some cognitive functions.
Areas 1,2,3,4,6 (primary sensorimotor area) → prefer putamen
Most intricately involved in coordinating motor movements.
The caudate and putamen are not directly connected to the thalamus. Output is mediated by the globus pallidus.
The output from the basal ganglia is not direct; it must first go through the thalamus, then back to the cortex.
The caudate and putamen receive the major input (afferents), and the pallidum send the major outputs (efferents).
The output from the basal ganglia goes mainly to the VA of the thalamus (and also to the VL, and the intralaminar nuclei).
The internal segment of the globus pallidus and pars reticulata of the subthalmic nucleus are similar in structure and function (output nuclei). The caudate and putamen are similar in structure and function (input nuclei).
Neurotransmitters:
Cortex to striatum = Glutaminergic/excitatory
Striatum to globus pallidus = GABA-ergic/inhibitory
All outputs from globus pallidus = GABA-ergic/inhibitory
Subthalamic nucleus to globus pallidus = Glutaminergic/excitatory
Striatum to subthalmic nucleus = GABA-ergic/inhibitory
Subthalmic nucleus pars compacta to striatum = Dopaminergic/excitatory
Movement results when the thalamic cells are released from tonic inhibition
Dopamine inhibits the indirect pathway and facilitates the direct pathway. Both actions serve to facilitate movement.
The Indirect Pathway
The second route by which information can get from the striatum to the globus pallidus, by way of the subthalamic nucleus
There is a two-way connection between the subthalamic nucleus and the globus pallidus. (These two structures are related, both derived from the diencephalon.)
Output from globus pallidus = GABA
Output from Subthalamic Nucleus = Glutamate
VI. The Pallidothalamic Connections (Fields of Forel and Associated Structures)
Ansa Lenticularis—formed by fibers that come from the ventral part of the medial globus pallidus and sweep around the posterior limb of the internal capsule on their way to the thalamus.
Forel Field H2 (lenticular fasciculus)—formed by fibers that come from the inner part of the medial globus pallidus, pass through the posterior limb of the internal capsule, around the zona incerta, and then on to the thalamus.
Forel Field H1 (thalamic fasciculus)—the ansa lenticularis and the lenticular fasciculus merge in the fields of Forel (H) to form this bundle of fibers, which then travel on together to the thalamus.
Most of the fibers terminate in the VA nucleus of the thalamus, some to VL, and some to the intralaminar nuclei.
The thalamocortical fibers project to area 6 (supplementary motor area, premotor, and prefrontal areas).
Zona Incerta—thought to be a rostral continuation of the midbrain reticular formation.
VII. Connections—Summary
The cortex sends fibers to:
Afferents to the striatum come from the:
Cortex
Substantia nigra
Others: Intralaminar nuclei (centromedian nucleus, parafasicular nucleus), dorsal raphe nucleus, reticular formation
Efferents from the striatum go to the:
Globus pallidus
Substantia nigra
Globus pallidus receives afferents from the:
Striatum
Subthalamic nucleus not from the cortex
Globus pallidus efferents go to the:
Thalamus (direct pathway)
Subthalamic nucleus (indirect pathway)
VIII. Clinical Application—Diseases of the Basal Ganglia
Carbon Monoxide Intoxication
Bilateral necrosis of the globus pallidus.
Other things that can cause bilateral lesions in the basal ganglia:
Cyanide, ethylene glycol (antifreeze)—both globus pallidus, methanol poisoning (putamen), aminoacidopathies, infarction, Hallervorden-Spatz syndrome, Huntington disease, Leigh disease, Wilson disease, mitochondrial encephalopathies, neoplasms (lymphoma, glioma), multiple system atrophy
Hallervorden—Spatz Disease
Autosomal recessive, childhood/adolescent onset, insidiously progressive
Clinical indications: stiff gait, toe-walking with arms held stiff and fingers hyperextended, distal wasting, pes cavus, frozen pained expression, speaks through clenched teeth, dystonic and bizarre postures
Caused by increased uptake of iron by the basal ganglia
T2-weighted magnetic resonance imaging: striking hypointensity of the medial segment of the globus pallidus, called the “eye-of-the-tiger” sign.
Path: hallmark is a golden-brown discoloration of the medial globus pallidus.
Hemiballismus
Most often resulting from a contralateral lesion of the subthalamic nucleus
Usually vascular cause
Fahr Disease—Basal ganglia calcification
“Status Marmoratus”
Perinatal damage to the striatum; results in glial scars that resemble marble
Involuntary movements, bizarre postures, spasmodic outbursts of laughing/crying. Intelligence may be normal (similar to choreoathetoid cerebral palsy)
Parkinson Disease
Huntington Disease
Wilson Disease
“Status Lacunaris”—arteriosclerotic Parkinsonism
Striatonigral Degeneration
Cranial Nerves
I. Introduction—Definition of Functional Components
Cranial Nerves (CN)
Special visceral afferent—afferent fibers related to special senses (smell and taste) concerned with visceral functions. Cell bodies lie in olfactory epithelium or in sensory ganglia.
Special sensory afferent—fibers related to special sense organs of the head (eyes and ears). Cell bodies are located in the retina and sensory ganglia of CN VIII.
Special visceral efferent (SVE)—motor fibers that supply all skeletal muscles derived from the pharyngeal arches. Cell bodies in the brainstem.
Spinal and Cranial Nerves
General somatic afferent (GSA)—fibers carry information from the body wall (i.e.,, somite-derived structures such as skin, skeletal muscle, joints, oral mucosa) to the CNS. Unipolar cell bodies lie in the spinal ganglia (dorsal root ganglia) and sensory ganglia of the CNs.
General visceral afferent—fibers carry information from viscera (such as heart, vessels, gut) to the CNS. Unipolar cell bodies lie in the dorsal root ganglia and sensory ganglia of the cranial nerves.
General somatic efferent (GSE)—motor fibers that supply all skeletal muscles of the body, except those derived from the branchial arches.
General visceral efferent (GVE)—autonomic motor fibers with preganglionic cell bodies in the CNS that send axons to peripheral autonomic ganglia. Fibers from postganglionic neurons innervate all smooth muscle, cardiac muscle, and glands throughout the body.
II. Cranial Nerve I—Olfactory Nerve
Function: smell—special visceral afferent
Pathways
Primary sensory neurons in olfactory epithelium → Pass through cribriform plate → Synapse on secondary neurons in the olfactory bulb (mitral and tufted cells) → Tufted cells synapse in the anterior olfactory nucleus and send projections to all olfactory areas, mitral cells send collaterals to the anterior olfactory nucleus and project only to the lateral olfactory area → Olfactory tract → Olfactory trigone → Olfactory stria
Olfactory Stria
Medial—projects to the frontal lobe (medial olfactory area). Mediates emotional response to odors and has connections to the limbic system.
Intermediate—projects to the anterior perforated substance.
Lateral—projects to the lateral olfactory area. The lateral olfactory stria have many important connections:
Lateral Olfactory Stria → Stria Medullaris → Habenula
Lateral Olfactory Stria → pyriform lobe, prepyriform cortex, periamygdaloid area, uncus, insula (primary olfactory cortex) → entorhinal cortex (secondary olfactory cortex)
Definitions
Pyriform lobe—parahippocampal gyrus, uncus, lateral olfactory stria
Prepyriform cortex—entorhinal cortex, uncus, insula, amygdala
Primary olfactory cortex—pryiform lobe and periamygdaloid area
Secondary olfactory cortex—entorhinal cortex
Diagonal band of Broca—connects the medial, intermediate, and lateral olfactory areas
Lesions
Anosmia—lack of smell. Occurs ipsilateral to the lesion and may be caused by:
Trauma—olfactory fibers are torn as they traverse the cribriform plate.
Frontal Lobe Masses—such as tumor, abscess. Caused by olfactory tract or olfactory bulb compression.
Hyposmia—decreased smell. Occurs commonly with:
Cystic fibrosis
Parkinson disease
Adrenal insufficiency
Cacosmia—repugnant smells. Can result from:
Damage to the olfactory area in the temporal lobe
Epilepsy—“uncinate fits”
Note: Olfaction is the only special sense that does not go through the thalamus.
III. Cranial Nerve II—Optic Nerve
Function: sight—special sensory afferent
Anatomy
The retina is composed of three cell layers:
Rods and Cones—photoreceptors that convert light waves into electrical impulses. Rods are responsible for night vision and are absent at the fovea. Cones are involved with color vision and acuity and are concentrated at the fovea.
Bipolar Cell Layer—also contains supporting cells such as Müller cells, horizontal cells, and amacrine cells. Bipolar cells receive impulses from the photoreceptors (rods and cones).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree