Fig. 2.1
CSF spaces and site of CSF production, circulation, and elimination (http://ihrfoundation.org/images/uploads/schematic_lg.gif)
The CSF space is regarded as one of the four compartments within the central nervous system (CNS) consisting of the vascular system, the brain parenchyma with extracellular space (ECS) and intracellular space (ICS), and the CSF compartment (Felgenhauer 1995) (Fig. 2.2).
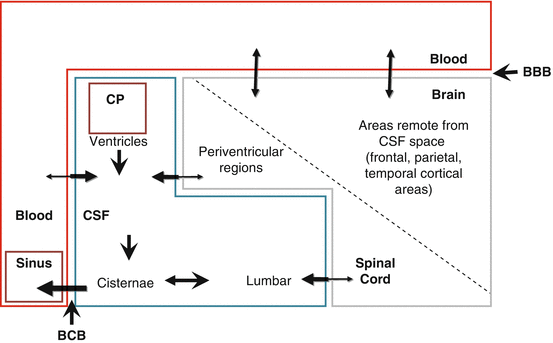

Fig. 2.2
The central nervous system (CNS) consists of compartments separated by blood-brain ( ) and blood-CSF (
) and blood-CSF ( ) barriers (BBB, BCB). Cerebrospinal fluid (CSF) is formed mostly at the choroid plexus and other sites along the BCB (80 %). Epithelia of the CSF space and the extracellular space of the CNS contribute a smaller fraction (20 %). Arrows indicate bulk flow within the CSF space and bilateral transfer processes across the barriers. CP choroid plexus
) barriers (BBB, BCB). Cerebrospinal fluid (CSF) is formed mostly at the choroid plexus and other sites along the BCB (80 %). Epithelia of the CSF space and the extracellular space of the CNS contribute a smaller fraction (20 %). Arrows indicate bulk flow within the CSF space and bilateral transfer processes across the barriers. CP choroid plexus
 ) and blood-CSF (
) and blood-CSF ( ) barriers (BBB, BCB). Cerebrospinal fluid (CSF) is formed mostly at the choroid plexus and other sites along the BCB (80 %). Epithelia of the CSF space and the extracellular space of the CNS contribute a smaller fraction (20 %). Arrows indicate bulk flow within the CSF space and bilateral transfer processes across the barriers. CP choroid plexus
) barriers (BBB, BCB). Cerebrospinal fluid (CSF) is formed mostly at the choroid plexus and other sites along the BCB (80 %). Epithelia of the CSF space and the extracellular space of the CNS contribute a smaller fraction (20 %). Arrows indicate bulk flow within the CSF space and bilateral transfer processes across the barriers. CP choroid plexusThese compartments of the CNS are separated by a barrier system (blood-brain barrier (BBB) and blood-CSF barrier (BCB)) which is important for maintenance of the cerebral environment and protection of the brain from the systemic circulation. The barriers are not completely impermeable, as assumed in earlier times based on the trypan blue experiments by Ehrlich and Goldmann, but permeable even for macromolecules and circulating cells (Davson 1976; Felgenhauer 1995). Both barrier systems allow an exchange between compartments next to each other, while the blood-brain barrier and the blood-cerebrospinal barrier differ both morphologically and with regard to their transfer properties (Abbott et al. 2010). A barrier between the parenchyma of the brain and the CSF compartment has not yet been defined. It is also unclear whether the protein content of the extracellular space differs from that in the CSF compartment (Davson et al. 1970).
2.2 The Blood-Brain Barrier (BBB)
The concept of an anatomical barrier separating blood and CNS first emerged from the studies of Goldmann (1913) who injected trypan blue into the venous system and observed that staining occurred throughout the body, whereas only the brain and CSF remained unstained. When he injected the dye into the CSF, the CNS tissue including the leptomeninges was strongly stained (Goldmann 1913). The role of this barrier system is to protect the brain from the outside environment and to maintain homeostasis of the brain (Dunn and Wyburn 1972; Abbott et al. 2010).
The special components of the BBB include capillary walls of endothelial cells, the basal membrane, and the perivascular layer of astrocytic end-feet. The BBB structures comprise a large surface area of 12–18 m2 in adult humans for exchange of humoral and cellular factors across this barrier (Abbott et al. 2010).
The capillary wall consists of a monolayer of non-fenestrated endothelial cells which form the functionally most important part of the BBB (Fig. 2.3) (Süssmuth et al. 2008). Where the endothelial cells overlap, their cell membranes are connected to each other by tight junction protein complexes known as zonulae occludentes. The tight junctions of the BBB consist of different integral membrane proteins including occludins, claudins, junctional adhesion molecules, and associated cytoplasmatic proteins (Koziara et al. 2006). The presence of tight junctions and the lack of fenestrae severely restrict paracellular transport. Accordingly, any transport of molecules to the brain must occur via the transcellular route by passive diffusion or active transport, which may be adsorption mediated, carrier mediated, or receptor mediated (Koziara et al. 2006).
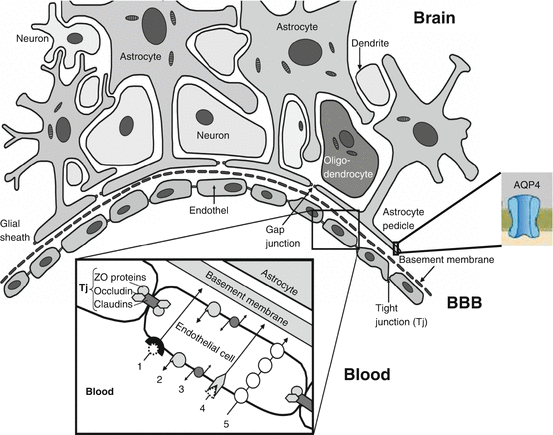

Fig. 2.3
Morphologic structure of the blood-brain barrier (BBB) and bidirectional transfer mechanisms (Modified according to Süssmuth et al. (2008)). 1 carrier-mediated transport, 2 efflux transport, 3 ion transport, 4 receptor-mediated transport, 5 transcytosis. T tight junction, ZO zonula occludens, AQP4 aquaporin-4
The endothelium basement membrane with a width of about 300–500 Ǻ offers no barrier to the passage of hydrophilic molecules such as ferritin (Brightman 1965). The vascular system and the neuronal system are not in direct contact but are covered by a sheath made up from processes of neuroglial cells including astrocytes and oligodendrocytes. The astrocytes dominate the transport route from capillaries to the neuron as seen in electron microscopy (Dunn and Wyburn 1972). Their processes variously called pedicles, end plates, or foot plates form a sheath covering the neurons, dendrites, axons, and capillaries. Another part of the satellite cells closely located to the neurons is the oligodendrocytes. Besides forming the myelin sheaths in the CNS, oligodendrocytes also take part in the formation of the glial sheath covering neuronal and vascular cells. A further element of the BBB is the extracellular space with a width of about 200 Ǻ that is labyrinthically ramifying between neurons, glial cells, and capillaries. It allows the unrestricted passage of ions and substances of colloidal size (Davson et al. 1970; Davson 1976).
Recent findings describe highly abundant AQP4 channels localized to perivascular and subpial end-foot membranes of astrocytes throughout the brain involved in regulation of extracellular space volume (Nagelhus and Ottersen 2013).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






