Fig. 1
Selection of significance value for voxel enhancement maps using T1-weighted spin echo brain MRIs in the cold-injury model. Significantly enhanced voxels after Gd administration appear white. (a) Representative pre- and post-Gd T1-weighted images for a sham rat and a cold injury rat. Area of injury in the cold injury model appears hyperintense post-Gd (blue arrow). (b) Signal intensities for post-Gd images were compared voxel-by-voxel using the Z-test with Bonferroni’s correction for multiple comparisons. The effect of testing for statistical significance at different probability values based on the number of voxels included in the multiple comparisons is shown. Groups of 100 voxels were selected for statistical comparisons based on a qualitative analysis of background noise and phantoms resulting in significance inferred for changes in signal intensities of voxels at p < 0.0005
NaF Histology for Cold-Injury Model
In sham animals, NaF enhancement was not observed at the surgery site (Fig. 2a). In cold injury animals, NaF enhancement was observed in the region surrounding the site of surgery (Fig. 2b); no enhancement was observed on the contralateral side. The median eminence, a circumventricular organ with a weak BBB, showed NaF enhancement in both sham and cold-injury animals (Fig. 2c).
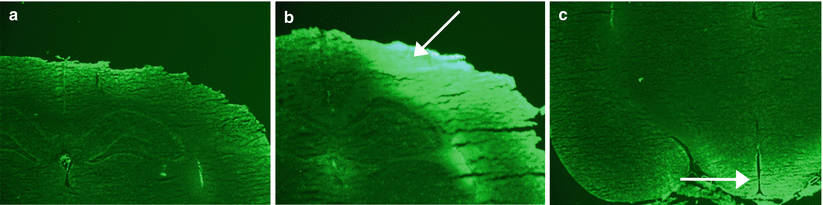

Fig. 2
Representative sodium fluorescein (NaF) histology for sham and cold-injury rats. (a) NaF histology for a sham rat with no obvious enhancement. (b) NaF histology for a rat that underwent cold injury. Intense focal enhancement is seen at the surgery site and adjacent area (white arrow). (c) NaF histology of a sham rat showing visible enhancement near the median eminence of the hypothalamus (area of weak BBB) (white arrow); this was seen for both conditions
Voxel-Based Analysis Shows Diffuse BBB Disruption in Low-Dose LPS and Mild Hypoxia
In control (Fig. 3a), low-dose LPS (Fig. 3b), and hypobaric hypoxia animals (Fig. 3d), no obvious areas of enhancement were observed. Furthermore, ROIs were drawn in the cortex, corpus callosum, and thalamus, and numbers of significant voxels were compared with controls. There was no significant difference (p > 0.05, one-way ANOVA with Dunnett’s test post hoc). The third ventricle, however, showed significant enhancement; the fraction of significant voxels in the hypoxia group was 0.11 ± 0.01, while controls was 0.08 ± 0.01 (mean ± SEM).
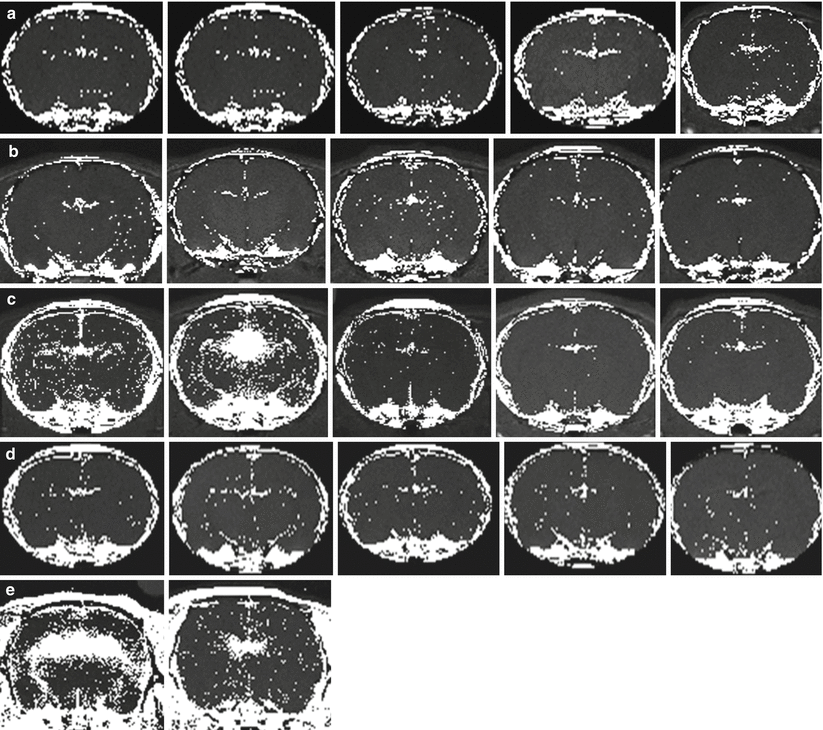

Fig. 3
Voxel-based analysis identified major enhancement in the periventricular area in the brains of rats exposed to high-dose lipopolysaccharide (LPS) and normobaric hypoxic conditions. Voxels with statistically increased signal after Gd administration are hyperintense. (a) Control rats that were exposed to 21 % O2. (b) Rats exposed to low-dose LPS (0.1 mg/kg). (c) Rats exposed to high-dose LPS (1 mg/kg). In two rats (leftmost images in the row), periventricular enhancement is obvious. (d) Rats exposed to hypobaric hypoxia (1/2 atm, equivalent to approximately 10 % O2 at sea level). (e) Rats exposed to normobaric hypoxia with 8 % O2. Periventricular enhancement was substantial in both rats. Statistical significance was set as p < 0.0005 using a Z-test
Voxel-Based Analysis Shows Enhancement of the Periventricular Area in High-Dose LPS and Severe Hypoxia
In high-dose LPS animals, two responses were observed (Fig. 3c). The first was seen in two animals and consisted of significant periventricular disruption (p < 0.0005, Z-test) accompanied by enhancement throughout the parenchyma. The second was seen in the other three animals, where there was no obvious disruption of the BBB. Thus, it appears that once a threshold has been reached, the BBB becomes disrupted throughout the brain with a focus on leakage in the periventricular area.
Rats exposed to 8 % normobaric hypoxia also showed significant enhancement in the ventricles and periventricular area (p < 0.0005, Z-test) (Fig. 3e). Furthermore, there were increased numbers of individual voxels showing Gd enhancement throughout the parenchyma in severe hypoxia.
Discussion
It is fairly straightforward to detect major localized BBB disruption such as that in tumors or stroke with Gd-enhanced MRI [17]. Such disruptions cause enough T1 signal change that regions can be seen without image processing. More quantitative methods, such as modeling the time course of enhancement or quantifying T1, lead to voxel-based data that, theoretically, can be used to detect changes in a single voxel. However, these methods require modeling assumptions, and it may be difficult to identify changes within one voxel with a high degree of statistical confidence.
The method proposed here does not include a modeling assumption. The main statistical limitation is that the high number of comparisons made in a single test can lead to a type 1 error (differences are detected when none exist). To minimize this problem, we examined BBB disruption in a cold-injury model where we knew there would be significant focal disruption. When using a probability threshold often used in biological studies (i.e., p < 0.05), most of the brain showed significant change. This may, in part, be due to the fact that the blood T1 was being shortened throughout the brain. The final p-value was selected to result in a statistical map that closely mapped the disruption observed using fluorescent microscopy. In the future, should this method be adapted for human use, such a threshold will require further validation and identification of an appropriate statistical threshold.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








