Examination component
Nonreassuring findings
1. Length of upper incisors
Relatively long
2. Relationship of maxillary and mandibular incisors during normal jaw closure
Prominent “overbite” (maxillary incisors anterior to mandibular incisors)
3. Relationship of maxillary and mandibular incisors during voluntary protrusion of mandible
Patient cannot bring mandibular incisors anterior to (in front of) maxillary incisors
4. Interincisor distance
Less than 3 cm
5. Visibility of uvula
Not visible when tongue is protruded with patient in sitting position (e.g., Mallampati class >2)
6. Shape of palate
Highly arched or very narrow
7. Compliance of mandibular space
Stiff, indurated, occupied by mass, or nonresilient
8. Thyromental distance
Less than three ordinary finger breadths
9. Length of neck
Short
10. Thickness of neck
Thick
11. Range of motion of head and neck
Patient cannot touch tip of chin to chest or cannot extend neck
Thanks to the improvement of electronics and optic fiber technology , many devices have been proposed in the last few years to overcome difficult intubation (Fig. 5.1). These devices have been compared with the classical MacIntosh laryngoscope and also with the fiberoptic bronchoscope or laryngoscope for their efficacy in improving visualization and in reducing neck movements and mechanical stress of cervical spine [25, 26].
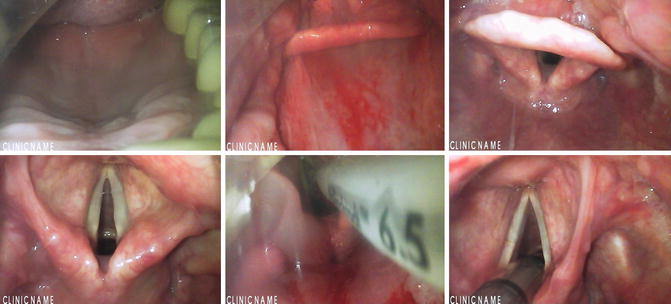

Fig. 5.1
Sequence of images during an endotracheal intubation with a GlideScope ®
Awake fiberoptic intubation can be performed using topic anesthesia with a conscious sedation in order to minimize coughing and neck movements [27]. This is the favorite technique for most practitioners in patients scheduled for general anesthesia with anticipated difficult intubation [28]. However, awake fiberoptic intubation is not without risks. In a closed claims analysis of 12 failed awake intubations, 9 cases (75 %) resulted in death or brain damage either for technical causes or for lack of patient cooperation, or development of airway obstruction for the sedation or edema [29].
Intraoperative Neurological Monitoring
Iatrogenic injury to the spinal cord and peripheral nerves could occur during CSS, caused from wrong positioning, surgical or anesthetic maneuvers or poor hemodynamic control. The blood supply to the medulla is granted from the anterior and posterior spinal arteries. The anterior spinal artery feeds approximately the two thirds of the cord mainly in the anterior and central area and the flow is centrifugal. The posterior spinal arteries feed the posterior part of the gray matter of the posterior horns and the more external portion of the anterior-lateral and posterior white matter, and its flow is centripetal. With the exception of the posterior half of the posterior horns, supplied only from the posterior spinal artery, the two systems have a discrete grade of overlapping. Unfortunately, the real efficiency of the interconnections is generally poor and not truly compensatory in case of obstruction of one of the two systems. Moreover, blood supply of the spinal cord is not homogeneous; the cervical tract is more vascularized with a good supply from both anterior and posterior systems, while thoracic and lumbosacral tract have respectively a weaker anterior and posterior flow [30].
In order to early detect neurologic modifications during spinal surgery, various neurophysiologic techniques have been proposed and used. Somatosensory-evoked potentials (SSEPs ) were the first to be studied and adopted. The registration of cortical or subcortical potentials after administration of peripheral stimuli and the evaluation of variations in amplitude and latency of the responses, helps in detecting possible functional impairment of the posterior afferent pathways. Typically the stimulating electrode are applied over the median nerve in the arm or over the posterior tibial nerve distally to the knee. The stimulation site is chosen depending of the site of surgery; when CSS is proposed the median nerve is generally used, while for surgery distal to the cervical segment the tibial nerve is stimulated [31].
In most cases, the technique gives indirect information about the functional situation of the anterior regions of medulla .
However, also for the anatomical and functional reasons described above, SSEPs may fail to detect spinal cord injury in the anterior-lateral area involving only the descending motor pathways without impairment of the posterior columns and gray matter.
TcMEPs monitoring was introduced to overcome these false negative responses. The electrical or magnetic stimulation of the precentral motor cortex with the peripheric recording under the surgery level of the muscular response can help assess the integrity of the anterior descending pathways.
Moreover, during CSS, TcMEPs and SSEPs seem to have different patterns of sensitivity: while TcMEPs are more useful to detect hypotension and cord hypoperfusion related injuries, SSEPs may be more helpful in preventing brachial plexus injuries [32].
Literature data highly recommend the continuous recording of both SSEPs and MEPs for the high sensitivity and specificity of the responses they can give when used together, allowing the recovery of situations which could otherwise have a very poor outcome. When pedicle screws are used, the intraoperative EMG is also recommended [33].
Special anesthetic care is needed when monitoring of somatosensory-evoked potentials (SSEPs) and/or motor-evoked potentials (MEPs) is planned, in order to detect intraoperative functional impairment of the spinal pathways. Anesthetic agents can heavily affect the quality of the monitoring, particularly for cortical SSEPs because of cortical direct depression. Moreover, general anesthesia causes a depression of intrinsic spinal cord activity, which is more evident when nitrous oxide or halogenated agents are used. Even if the use of trains of stimuli rather than a single one tends to overcome this poor excitability, the depressive effect is still significant .
Hence, the simultaneous monitoring of a cortical and a subcortical site of SSEPs may help, when necessary, in the interpretation of a decrease in cortical SSEPs amplitude and/or increase in latency, because the subcortical response is far less impaired by the anesthetic effect. Generally the first choice should be a TIVA, because of the impact of inhalational anesthetics on evoked potentials even at low concentrations [34]. Propofol suppresses the activity of the anterior horn cells, but significantly less than halogenated anesthetics [35]. Also intravenous drugs should be chosen carefully: benzodiazepines and barbiturates produce CMEPs depression at doses less than those affecting the SSEPs and lasting for several minutes. Opioids are an important component of anesthesia for evoked potentials monitoring: they only produce minimal changes in spinal or subcortical SSEP recordings, a mild decrease in amplitude and increase in latency for cortical SSEPs and myogenic responses from MEPs [36].
Recently it has been noted that also remifentanil, when used at higher doses, can affect SSEPs monitoring, acting particularly on the amplitude of signals [37].
Spinal MEPs (stimulating cranially to the level of surgery) or pedicle screw testing during spinal instrumentation (EMG recording) are virtually insensitive to anesthetic agents, while could be hindered by muscle relaxant drugs. However, a controlled degree of neuromuscular blockade with two twitches remaining in the train of four allows an effective monitoring and is preferred in order to avoid excessive movements and facilitate surgery. Many Authors use a continuous infusion of a muscle relaxant agent, generally cis-atracurium, titrated to obtain and maintain the desired pattern of train-of-four.
In any case, due to the complex pattern of interference between anesthesia and intraoperative neurophysiologic monitoring, a continuous exchange of information among all the practitioners involved can improve the interpretation of data and the outcome of the patient [36].
Patient Positioning and Related Complications
Positioning in CSS is potentially challenging. A study on 75 patients undergoing CSS with IONM showed a sudden worsening during positioning of trans cranial MEPs in three cases and both MEPs and SEPs in two cases. Despite the immediate adjustment of the patient’s position and the stabilization of an adequate blood pressure, in one case evoked potentials remained depressed during surgery and the patient presented delayed neurological impairment in the postoperative (tetraparesis), but fortunately with a complete recovery after 2 weeks. The other four patients gradually showed improvement of evoked potentials after re-positioning with no neurological deficits at the end of surgery [38].
Neurological impairment, mostly transitorial, is also reported after non-cervical surgery particularly in the elderly patients in which unsuspected cervical stenosis are often present [39]. This always suggests cautious positioning of the head and possibly, in every case of supposed spinal cord compression, a proper maintenance of mean arterial blood pressure that may have potential benefit in improving the blood supply to ischemic areas [30].
Peripheral nerve injury is a rare complication after surgery, generally caused from bad patient positioning with an overall rate ranging from 0.03 to 0.1 % [40]. The complication seems more frequent in patients with some comorbidities such as diabetes mellitus, alcohol addiction and vascular disease, and particularly in the elderly and in the extreme ranges of body mass index. Literature data are poor and missing in randomized trials about the matter; no guidelines are available to help in choosing the correct positioning in any kind of surgery; only some advices have been proposed based on expert opinions, case reports and consensus surveys. The abduction of the arm seems to be more tolerated in the prone rather than in the supine position, though it’s advised not to exceed 90° [40]. In the supine position with the arm abducted the ulnar nerve is better protected with the forearm in supine or neutral position; when the arm is tucked at side the forearm should be in neutral position and in any case pressure on the ulnar groove at the elbow and on the radial spiral groove of the humerus must be avoided. Flexion of the elbow may increase the rate of ulnar impairment, while excessive extension beyond the range preoperatively assessed as comfortable may stretch the median nerve. During surgery the position of the upper extremities should be periodically reassessed. Gel or foam padding are advised but they must be used carefully, possibly by experienced staff. A wrong use of padding can even increase rather than decrease the rate of postoperative neuropathy [41].
One of the most devastating complications in non-ocular surgery is the PeriOperative Visual Loss (POVL), in some cases caused from wrong position. POVL is rare if considered in the whole population of surgical patients, ranging from 1:60.000 to 1:125.000, but is more frequent in spine surgery (3.09:10.000); only cardiac surgery has a higher risk of POVL (8.64:10.000). The causes of POVL are mainly two: the Central Retinal Artery Occlusion (CRAO) and the Ischemic Optic nerve Neuropathy (ION). The CRAO leads to the ischemia of the entire retina, while the less severe obstruction of a branch of the artery (BRAO) causes an impaired function only in a sector. Whereas during cardiac surgery the most common mechanism involved is the arterial microembolism, during spine surgery the complication mainly derives from an improper head position, leading to mono or bilateral ocular compression [42]. Recently, an ASA task force has proposed some practical advices for POVL prevention in spine surgery. For the prevention of CRAO and other ocular damage direct pressure on the eye should be avoided, the eyes of prone-positioned patients should be assessed regularly and documented [43]. ION is less rare than CRAO, accounting for about 89 % of cases of POVL after spinal surgery. The mechanisms underlying the development of ION are not completely known, but the pathogenesis seems to be multifactorial [42]. The occurrence of ION seems to be strictly correlated with surgery duration. In a survey of 83 ION after spine surgery, the majority of the cases (94 %) occurred for 6 h anesthetic duration or longer, while only one case was associated with surgery lasting less than 4 h [44]. Other risk factors for ION were detected such as obesity, male sex, Wilson frame use, greater than estimated blood loss, and decreased percent colloid administration. The pathogenesis of ION is not clear. The most popular theory involves the elevation of venous pressure and the development of interstitial edema leading to deformation and obstruction of the vessels feeding the optical nerve. All the factors able to increase the venous pressure in the head or to decrease the oncotic pressure could predispose to ION. Some examples are the prone position with abdominal compression in obese patients or the head position lower than the heart for the former; a significant blood loss with consequent hypoalbuminemia and the scarse administration of colloids for the latter [45].
Other complications deriving from improper positioning should be prevented using gel or foam-made dedicated devices (Fig. 5.2) or even normal pillows put together with the active contribution of the surgeons, the nurses and the anesthesiologist. The final result must ensure the distribution of the pressures as more as possible over larger extensions of tissues, avoiding excessive and localized compressions, and excessive stretching or flexion of elbows, shoulders and neck. Abdomen compression should be avoided to facilitate intermittent positive pressure ventilation and limit barotrauma. Moreover, the reduction of the intrathoracic mean pressure leads to improvement of venous return and helps in lowering surgical bleeding. This is particularly important in CSS, where a deliberate arterial hypotension must be generally avoided to ensure a proper blood perfusion to the spinal cord. As discussed above, the head and the face should be frequently checked to avoid harmful compressions on the eyes and ears (Figs. 5.3 and 5.4) [46].

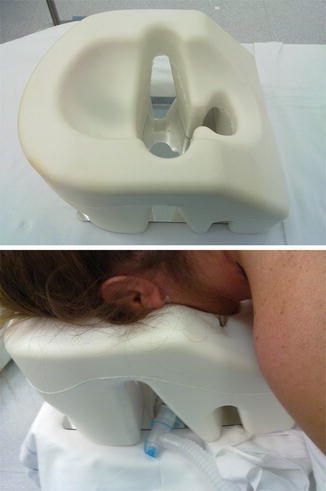
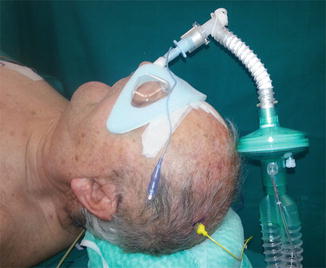

Fig. 5.2
Gel padding devices

Fig. 5.3
A foam-made headrest for prone positioning, with a mirror for eyes check

Fig. 5.4
Nasotracheal intubation for ACSS at C3 level. The eyes are protected by an adhesive shell-shaped device
Prophylaxis of Surgical Site Infection
Surgical site infection (SSI) is a dreadful and costly complication in spinal surgery. A retrospective study regarding 90 patients undergoing posterior CSS showed no infections in upper cervical surgery (all infected patients were operated at C3 level or below) while the use of a rigid collar in the postoperative is underlined as an important risk factor for infections of the wound in subaxial cervical surgery [47]. Other known risk factors were investigated, such as smoke with an odds ratio (OR) = 2.10 and perioperative steroids (OR = 3.42), but neither resulted statistically significant. A larger series of 318 patients undergoing posterior cervical decompression, showed an incidence of 1.6 % for SSI needing reoperation (5 cases) with a statistically significant correlation between postoperative infection and the number of levels decompressed [48]. In a retrospective study on 1,615 lumbar spine fusions (1,568 patients), the overall rate of infection in was 2.2 %. Risk factors detected were diabetes (×6), smoke (×2) and positive history of spinal surgery (×3.7). Moreover, risk increased with the number of levels fused [49].
Literature data support the efficacy of perioperative antibiotic prophylaxis in all the orthopedic spinal procedures with or without instrumentation, with a grade A in the strength of recommendation. The standard recommended agent is cefazolin 2 g i.v. for adult patients (3 g in patients weighting over 120 kg, 30 mg/kg for pediatric patients), administered within 60 min before skin incision. Clindamycin or vancomycin should be used as alternative agents in patients with β-lactam allergy. If organizational SSI surveillance shows that gram-negative organisms are associated with infections or if there is risk of gram negative contamination of the surgical site, clindamycin or vancomycin should be used in addition to cefazolin if the patient is not β-lactam allergic, or to aztreonam, gentamicin, or single-dose fluoroquinolone if the patient is β-lactam allergic. In patients who are known to be colonized with methicillin-resistant Staphylococcus aureus (MRSA), vancomycin should be added to cefazolin. For agents requiring a slow infusion over 1–2 h, as fluoroquinolones or vancomycin, the administration should begin within 120 min before skin incision. For patients with renal or hepatic impairment, the dose often does not need to be modified when given as a single preoperative administration before surgical incision. In order to maintain an adequate blood and tissue drug concentration, redosing is recommended when the duration of the procedure exceeds two half-lives of the drug or there is excessive blood loss [50].
Deep Venous Thrombosis and Pulmonary Embolism Prevention
DVT complicates CSS with a mean rate of 0.5 %, with an higher incidence after posterior fixation (1.3 %) than after anterior CSS or posterior decompression (<0.5 %). Despite this low rate of occurrence, the hospital stay increases by 7- to 10-fold over normal when DVT is present, and mortality rates increase by 10- to 50-fold [51]. In a prospective clinical trial in patients undergoing CSS, mechanical prophylaxis with intermittent pneumatic compression (IPC) was equally effective as unfractioned heparin or low molecular weight heparin for the prevention of DVT and PE, but avoided the risk of postoperative hemorrhage [52].
The 9th edition of the Antithrombotic Therapy and Prevention of Thrombosis Guidelines from the American College of Chest Physicians suggests mechanical prophylaxis, preferably with IPC, over no prophylaxis or pharmacological prophylaxis. For patients undergoing spinal surgery at high risk for Venous ThromboEmbolism (VTE), including those with malignant disease or those undergoing surgery with a combined anterior-posterior approach, the guidelines suggest adding pharmacologic prophylaxis to mechanical prophylaxis once adequate hemostasis is established and the risk of bleeding decreases [53].
Postoperative Pain Management
Pain after spine surgery is often more severe than in other surgical settings. Skin incision frequently involves multiple adjacent dermatomes and painful anatomical structures are often involved as periosteum, ligaments, facet joints, muscular fascial tissue. Among the deep somatic structures, periosteum seems to be one of the most painful tissues having the lowest pain threshold nerve fibers [54]. Complex mechanisms of peripheral and central sensitization of pain receptors and spinal cord pathways are also involved in explaining the resistance to treatment and the tendency of the pain to persist even after days. In addition, patients scheduled for spine surgery are often under preoperative chronic pain therapy. In some patients, a large use of opioids in the preoperative creates serious therapeutic challenges in the postoperative, making pain less responsive to incremental doses of opioids [55].
When minimally invasive techniques are adopted, pain could be reduced for the generally small skin incisions and the reduced damage for muscles and deep tissues. However, among the postoperative “side effect” of surgery, pain represents one of the most common causes of hospital re-admission or delayed discharge, especially when an outpatient (OP) or day surgery (DS) treatment is planned. Nowadays, the multimodal approach to pain therapy is considered the best model of treatment, because it allows to reduce the doses of the single drugs used and to minimize the potential side effects. The multimodal or balanced treatment consists in combining opioid and non-opioid analgesics with additive or synergistic actions since the preoperative period [56].
Other techniques can be adopted together with drug therapy to help to decrease postoperative pain. Skin and tissues infiltration with a long acting local anesthetic added with epinephrine before the surgical incision is a common practice. This technique reduces intraoperative bleeding and analgesics requirement, at least in the earlier postoperative period. Continuous postoperative wound infiltration with local anesthetics through microcatheters of various length is also available, but not so widely used, even if the efficacy and the low rate of complications have been demonstrated [57, 58].
Due to the large margin of safety and the very rare complications , acetaminophen deserves a special place in the management of pain after CSS. Acetaminophen alone could efficiently control a moderate pain. When used in combination with other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) or weak opiates, it significantly reduces the consumption of other analgesics in the postoperative. The availability of oral and intravenous (iv) preparations makes acetaminophen suitable both for perioperative and for postoperative use, and also allows to continue the support therapy easily after patient discharge [56].
NSAIDs and cyclooxygenase-2 inhibitors (COX-2 ) lead to increased risk of non-union after spine fusion surgery, but this adverse effect seems limited to prolonged use (>14 days) or high doses. The use of ketorolac at a dose of more than 120 mg/day even for few days or the use of a cumulative dose of more than 300 mg of diclofenac significantly affect the risk of non-union [59]. When used at lower doses and for few days, these drugs surely help in postoperative pain treatment.
Opiates still have an important role in the treatment of moderate-to-severe postoperative pain, but because of the important side-effects, it’s advisable to reduce the doses in a multimodal protocol. The association of NSAIDs or celecoxib with a slow-release oxycodone since the preoperative period improved the outcome of spine surgery when compared with intravenous morphine, providing earlier recovery of the bowel function [60]. Patients treated preoperatively with opiates for chronic pain could necessitate large opiates doses in the perioperative period. The use of intraoperative ketamine infusion in these patients has significantly lowered opiates consumption even over 6 weeks after spine surgery, particularly after CSS. The clinical benefit in terms of reduction in opiate-related PONV has been higher for CSS than for lumbar surgery, while the ketamine related side-effects such as disturbing dreams and hallucination were more common after lumbar surgery [61, 62].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








