CHAPTER 20 Anesthetic and Intensive Care Management of the Patient with a Meningioma
ANESTHETIC CONSIDERATIONS
Preoperative Evaluation and Preparation
When intracranial procedures are planned, the preoperative evaluation must address various physiologic aspects of the patient’s condition. Neurologically there must be documentation of the patient’s level of consciousness, cranial nerve function, neurologic deficits, and the presence or absence of elevated intracranial pressure (ICP). Patients with a history of hypertension, cardiovascular disease, cerebral vascular insufficiency, or previous carotid endarterectomy may have altered levels of cerebral autoregulation, impaired cerebral perfusion, or abnormal baroreceptor function.1–3 Intravascular volume depletion may be a result of diminished oral intake from nausea and vomiting, preoperative osmotic diuresis, and even the use of intravenous contrast administration for diagnostic studies.
Choice of Patient Position
Complications related to maintaining abnormal positions may occur. Extreme care is required regarding prophylactic padding. Hyperflexion–extension of the head is to be avoided. Head flexion can result in oropharyngeal complications. Compression by artificial airways and endotracheal tubes can occur.4 Leg pneumatic venous compression devices are used to relieve the incidence of deep venous thrombosis (DVT) from prolonged immobilization. This is true for all neurosurgical interventions, but is particularly important in patients with meningiomas, as these patients often present with a hypercoagulable state and are prone to the development of DVT.
Prone
A dreaded complication of the prone position is retinal ischemia and blindness. The mechanism is unknown, although orbital compression, low arterial blood pressure, and poor venous drainage have been suggested. Prolonged procedures greater than 7 hours seem to be an important factor.5
Lateral
The lateral position can be an alternative to the sitting or prone position. A vacuum mattress conforms to the patient’s anatomy. The patient must not move after head fixation. This can cause strain in the cervical area.6 The lateral position is used particularly for approaches to the cerebellopontine angle, and for skull base approaches to the foramen magnum, petrous bone, and petroclival junction. It is the most complex positioning and requires great attention to detail to prevent axillary compression, brachial plexus stretch injuries, vascular compromise, and adequacy of monitoring placement.
Sitting
The term sitting is a misnomer. The patient is in a modified recumbent position, as shown in Figure 20-1A and B. The legs are high to promote venous return and to elevate central venous pressure (CVP). This enhances circulatory stability and may reduce the chance for air embolism. Modifications to the sitting position permit lowering of the head without taking the patient out of the head holder. This is important if venous air embolus (VAE) is suspected because it allows rapid lowering of the head in a critical situation.
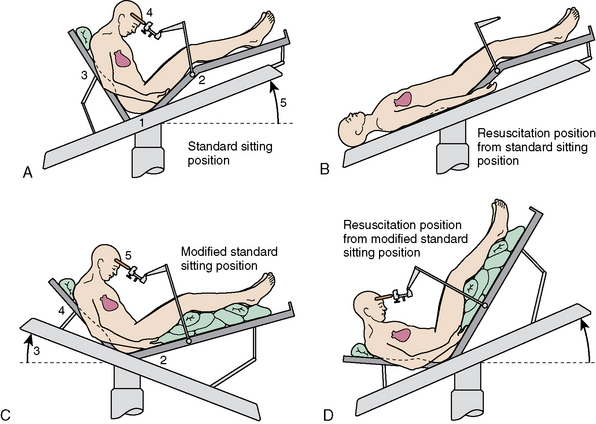
FIGURE 20-1 A–D, Variations of sitting position.
(From Miller RD, ed [2004]. Anesthesia, 4th ed, chapter 56, p. 1900, figure 56-1. Philadelphia: Churchill Livingstone, reproduced with permission.)
Head flexion is required to improve access to posterior structures. A two-fingerbreadth distance between the chin and the chest must be maintained to prevent compression ischemia. The bite block or oral airway must be positioned to prevent pressure on the base of the tongue. Large increases in ICP can occur with extreme head flexion and rotation or the addition of the positive end-expiratory pressure (PEEP).7 Head flexion may also result in downward migration of the endotracheal tube down the right mainstream bronchus. The positions shown in Figure 20-1C and D permit surgery to continue with the head to heart venous pressure gradient dimensions. Only the position shown in Figure 20-1D is compatible with closed-chest cardiac massage.
The sitting position for neurosurgery remains controversial. Its use has been diminishing because of potential serious complications, although many surgeons believe it is highly advantageous. The sitting position may be used because it provides better access to midline lesions, improved cerebral venous decompression, lower ICP, and gravity drainage of blood and CSF. The latter minimizes the need for exhaustive cautery with improved preservation of arachnoid plains. The sitting position also facilitates direct observation of facial musculature as a means of determining irritation of the facial nerve. Intraoperative facial electromyography improves direct observation by providing a continuous and more sensitive monitor of facial nerve functions.1,8
Complications related to the use of the sitting position include venous air embolism (VAE), paradoxical VAE, circulatory instability, pneumocephalus, subdural hematoma, compressive peripheral neuropathy, quadriplegia, and skin compressive lesions.9 The severity of these complications have made most neurosurgeons in the United States abandon the position, while it is still used extensively in other parts of the world, particularly in Europe. Mild transient postural hypotension (–20 to –30 mm Hg) occurs in about one third of anesthetized patients placed in the sitting position. Marked hypotension (–50% of supine values) occurs in 2% to 5% of cases.10,11 Patients with heart failure or severe coronary/or cerebral occlusive vascular disease are a relative contraindication to the sitting position.
Retrospective reviews of surgeons who performed procedures in the sitting and various horizontal positions concluded that each position has its own benefits and risks. They found no support of increased morbidity or mortality with either position.12,13 In one series, although VAE occurred more than three times as often in seated patients as in the horizontally positioned patients, no increased clinical complication rate was found. Seated patients lost less blood and required fewer blood transfusions when compared with supine, prone, and lateral patients.13
Venous Air Embolism
Venous air entrainment (VAE) results from both an open vein and negative intravenous pressure relative to atmospheric pressure. It occurs when the head is positioned above the heart to encourage cerebral venous drainage. One study found VAE incidence rates of 25%, 18%, 15%, and 10% associated with sitting, lateral, supine, and prone positions, respectively. Low CVP and poor surgical techniques increase VAE incidence. With the use of a precordial Doppler monitor, the VAE incidence ranges form 25% to 50% during suboccipital craniotomy.14–16
The highest risk portion of the operation for VAE is during skin–muscle incisions and when bone venous sinusoids are exposed during the dissection.9 Highly vascular lesions also predispose to VAE. VAE is most severe when a dural sinus is open, and therefore skull-base approaches, with their exposure of multiple venous sinuses, are particularly prone to VAE. It may also occur from headholder pins, burr holder, and connections in venous catheter systems.9
The clinical significance of VAE is influenced by several factors, including the volume of intravascular gas, its rate of entrainment, the presence of a patent foramen ovale, elevated right heart pressure, the presence of nitrous oxide, anesthetic depression of cardiovascular function, and the patient’s cardiopulmonary compensatory capacity. Small bubbles of air entrained slowly are of little physiologic significance. The venous gas bubbles are removed by the lungs at a rate that depends mainly on a compensatory rise in pulmonary artery pressure (PAP).17,18 The PAP plateaus as the rate of venous entrainment of the gas equals the rate of its pulmonary excretion. At this point equilibrium occurs. If this excretion capacity overloads it leads to further PAP increases, pulmonary shunting, and reduced cardiac output and circulatory collapse. VAE-reduced cardiac output and/or increased dead space leads to a decrease in end-expired carbon dioxide, making end-tidal carbon dioxide monitoring extremely useful in this setting. A fall in end-tidal CO2 is the first sign of a VAE. Mild carbon dioxide retention occurs as dead space increases in embolized regions. The diagnosis of VAE may be confirmed by a blood gas measurement. A large end-tidal carbon dioxide to PaCO2 gradient in the absence of a recent change in controlled ventilation results. A late occurrence during an air embolism is hypoxemia, which occurs as a result of the shunting of pulmonary blood flow.18
Chronic postoperative perfusion deficits associated with increased pulmonary vascular permeability have been seen with small amounts of air over a prolonged period.19,20 Acute respiratory distress syndrome may result.
Paradoxical air embolism is another serious complication of VAE. With a patent foramen ovale, intravenous gas may pose to the left side of the heart and lodge in the brain, heart, or other vital organs. A patent foramen ovale and elevated right heart pressure occurs in about 5% to 10% of adult neurosurgical patients.21 A transesophageal echocardiogram (TEE) can be used to detect the presence of left-sided aerated saline.22 The operative TEE, however, is of limited value and can only provide guidance of a positive test result. In the presence of a known patent foramen ovale, a position other than seated should be used. PAP monitoring can detect a right-to-left arterial pressure gradient. It may reveal a paradoxical VAE.23 Volume loading to elevate left atrial pressure or lowering the table can be helpful. PEEP elevates cerebral venous pressure and may reduce the potential for VAE, but its use remains controversial. Its ability to reverse the normal PAP to left atrial pressure gradient remains unclear.24
When nitrous oxide is used as part of the anesthetic technique, the volume of entrained intravascular gas is increased.22 Nitrous oxide is 34 times more soluble in blood than nitrogen. It rapidly diffuses into an intravascular air bubble. It has been suggested that reducing the inspired concentration of nitrous oxide enhances safety.25 A clinical study comparing 50% nitrous oxide with nitrogen showed that the use of 50% nitrous oxide has no effect on clinical outcome in the presence of minor episodes of VAE.16
The surgeon should be informed and the prevention of additional air entry should be prevented. The wound should be packed, and cerebral venous pressure should be increased by applying jugular venous compression or by lowering the patient’s head. Pharmacologic cardiovascular support should be used if deterioration occurs and the patient should be placed supine and resuscitation should proceed. The left lateral decubitus position may remove air from the pulmonary artery back into the right ventricle.5,26
The sitting position promotes CSF drainage form the intracranial space with resultant air entry and consequent pneumocephalus. Some degrees of pneumocephalus can occur in any craniotomy, but is accentuated in procedures performed in the sitting position.27 Air entry is further facilitated by surgical decompression, diuretics, and hyperventilation. Air can remain trapped inside the skull when the dura is closed and can produce a mass effect. This mass effect may be asymptomatic or may be associated with headache, confusion, impaired memory, and lethargy. The symptoms usually resolve over 4 days.9 Breathing 100% oxygen will hasten reabsorption of the pneumocephalus.
Cervical spinal cord ischemia due to neck flexion can result in quadriplegia.28 Hypotension in the sitting position could potentiate this injury. Patient should be questioned perioperatively about upper extremity paresthesia related to neck position. Vigilance should he exerted to avoid extreme neck flexion, which in addition to cord ischemia can obstruct venous return resulting in marked swellings of the head and tongue. Small oral airway and late blocks should be used to ensure jugular venous patency.9 One should consider evoked potential monitoring to detect intraoperative cervical spinal cord ischemia.
Padding should be used in dependent areas and anatomically correct positioning should be maintained. Stretching the sciatic nerve and compression ischemia of the nerves and skin should be avoided.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








