, Nima Etminan1 and Daniel Hänggi1, 2
(1)
Neurochirurgische Klinik, Universitätsklinikum Düsseldorf, Düsseldorf, Germany
(2)
Medical Art Christine Opfermann-Rüngeler, Zentrum für Anatomie Heinrich Heine Universität, Düsseldorf, Germany
6.1 Anterior Communicating Artery Aneurysms
6.1.1 General Considerations
As described earlier in this book, anterior communicating artery aneurysms are best classified on hemodynamic grounds into four types according to the projection of the dome. The projection of the dome determines to a large degree specific problems of the approach and the manner of clip application:
Type 1 aneurysms project downward to the optic chiasm and often adhere to this structure. This adherence is important because elevating the orbital cortex with a brain retractor during the approach may lead to traction on the aneurysm dome and to premature intraoperative rerupture.
Type 2 aneurysms project essentially forward as an elongation of the dominant A1 segment. Although they do not adhere to the chiasm, they lie within the interhemispheric fissure, so formal splitting of the fissure between the gyri recti is therefore hazardous with these aneurysms.
The dome of type 3 aneurysms lies approximately within the plane of the A2 segments. Although the initial approach is less problematic than with the type 1 and 2 projections, type 3 aneurysms require more upward dissection and retraction, owing to the higher position of the aneurysm neck. Furthermore, type 3 aneurysms are close to the perforators originating from distal A1 and proximal A2 segments.
Type 4 aneurysms lie above the plane of the A1 segments and behind the plane of the A2s. These aneurysms require access to the neck above the A1s, which is between perforators. Type 4 aneurysms are also close to the hypothalamus, and this structure is hurt by aneurysmal hemorrhage and surgical manipulations more easily than with the other projections. Therefore, type 4 projections are associated with a less favorable functional outcome than the other projections.
Of course, the other important variable that determines the complexity of surgical obliteration and outcome is aneurysm size. It is unclear whether the effect of size on surgical problems and outcome is different than for other anterior circulation sites, but it appears likely that this is indeed the case. That is, giant anterior communicating artery aneurysms have a worse outcome than giant middle cerebral artery aneurysms.
6.1.2 Dissection of Anterior Communicating Artery Aneurysms and Clipping
The orbitocranial approach is favored for all anterior communicating artery aneurysms, as outlined earlier. Prior to opening the dura, sufficient brain relaxation is achieved by drainage of 50–100 mL of CSF, in case of subarachnoid hemorrhage (SAH) by a lumbar or ventricular drain and by mannitol if necessary (1–2 g/kg body weight). Following gentle elevation of the orbital cortex and opening the central aspect of the sylvian fissure, further steps depend on the aneurysm projection. In any case, the next waypoint is the optic nerve. The small retractor blade that is usually necessary for this approach must be pointing to the ipsilateral optic nerve. This strategy prevents severing the aneurysm with the retractor blade because the anterior communicating artery and the aneurysms almost always lie completely in front of the optic nerve. Nonetheless, care is mandatory even when the retractor blade is not put in front of the chiasm, particularly with a type 1 projection, where the dome of the aneurysm often adheres to the anterior aspect of the chiasm.
Following insertion of the blade, the prechiasmatic cistern is opened medial to the now-opened sylvian fissure. Finally, the cistern must be opened all the way medially to the interhemispheric fissure to allow mobilization of the straight gyrus and access to the A1 segment. At this stage, the surgeon must decide how to proceed. Medial opening of the prechiasmatic cistern and splitting the proximal interhemispheric fissure can be done safely with aneurysm projections type 3 and 4. The interhemispheric arachnoid adhesions can be separated by using bipolar forceps with a spreading motion, as usually done to split the sylvian fissure [1]. Care is required with ventral (type 1) and anterior (type 2) projections. A straight anterior projection of the aneurysm dome renders formal splitting of the interhemispheric fissure dangerous. In these situations, it may be more appropriate to perform a small resection of the gyrus rectus medial to the olfactory tract.
These steps allow further mobilization of the orbital cortex. The posterior aspect of the gyrus rectus is now separated from the chiasm. The A1 segment of the anterior cerebral artery must be identified at this stage. The artery should be looked for at the point where it crosses the optic nerve (Fig. 6.1). It is not necessary to follow the artery to its origin from the internal carotid artery. When the artery has been identified, the back side must be dissected locally in order to apply a temporary clip, in case clipping should become urgently necessary. Attention must also be paid to the retrograde Heubner’s artery, which originates on the ventrolateral aspect of the A2 origin and then retrogradely parallels the A1 segment along its dorsal circumference.
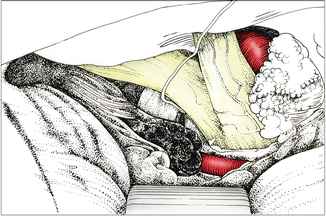

Fig. 6.1
Ventrally projecting anterior communicating aneurysm (type 1) approached from the right. Identifying A1 at the level of the optic nerve
The exposure can now be stabilized prior to further dissection. To distribute the pressure on the orbital cortex, it is helpful to insert a rolled cottonoid in front of the spatula between the orbital roof and the orbital cortex. During this maneuver, attention must be paid to the olfactory bulb.
A few words shall be spent regarding opening of the lamina terminalis, which now comes into the surgical field. If there is functioning ventricular or spinal CSF drainage, opening the lamina terminalis does not add much with regard to brain relaxation. If operative access has been achieved without external CSF drainage, opening the lamina terminalis certainly helps to relax the brain. Other benefits have been postulated, such as minimizing postoperative hydrocephalus or vasospasm, but convincing proof has not been provided.
The A1 is now followed anteriorly to the anterior communicating artery. With high-running A1s, a certain problem often occurs at this stage. It may become necessary to further split the interhemispheric fissure or to perform some medial resection of the straight gyrus. A limited resection is not known to have any negative consequences. Applying undue retraction with the retractor blade, on the other hand, must be avoided not only because of brain damage but also because it is usually a futile effort to achieve sufficient exposure in cases of insufficient dissection.
Following identification and dissection of the A1-A2 transition, the further steps depend essentially on the type of projection. If it is easily possible, the surgeon should now attempt to achieve complete control of afferent blood flow—that is, to identify the contralateral A1 and prepare it for temporary clipping. In case of type 1 projection, however, this is usually not feasible and should be omitted. With the other projections, however, the contralateral (usually hypoplastic) A1 should be controlled at this stage. The direction of the contralateral A1 is usually straight up toward the surgeon. The artery should be dissected segmentally in order to apply a temporary clip. The origin of the contralateral A2 is commonly hidden behind the aneurysm; it is therefore difficult to identify at this stage, and no effort should be made to do so. Identification of the contralateral A2 becomes easier after dissection of the aneurysm neck. Sometimes, however, the contralateral A2 can be visualized only after applying a clip on the aneurysm neck. During dissection of the A2 segments, the origin of Heubner’s artery needs special attention and must be spared.
It is worthwhile to quickly review the specific pathoanatomy with regard to the A2 segments. Physiological elongation of the A1 segments during aging leads normally to a rotation of the anterior communicating artery so that the origin of A2 on the side of the larger A1 is displaced posteriorly. In our experience, this configuration is found in approximately 80 % of all patients with an anterior communicating artery aneurysm. In the remaining 20 %, however, the origin of the A2 is displaced anteriorly on the side of the dominant A1. As nicely pointed out by Suzuki and colleagues [2], this configuration poses an additional surgical difficulty as the contralateral A2 is hiding behind the ipsilateral one. This so-called closed configuration results also in a distinctly worse functional outcome than with the so-called open A2 configuration, in which the contralateral A2 can be easily identified in front of the ipsilateral A2.
Now that proximal control has been gained, the further steps are dissection of the aneurysm neck and clip application, as outlined earlier. With inferiorly projecting aneurysms (type 1), both sides of the neck are dissected in front beneath the anterior communicating artery (Fig. 6.2). With anterior projecting aneurysms (type 2), necessary neck dissection may be limited to the space in front of the anterior communicating artery (Fig. 6.3), but sometimes with a wide neck, the origins of the A2s stick to the neck and must be freed. As mentioned, identification and separation of the contralateral A2 may not be easy at this stage, and the situation may need clarification after applying a pilot clip on the aneurysm neck. Corresponding to the dissection, both clip branches are then passed beneath or in front of the anterior communicating artery, essentially parallel to the plane of the anterior communicating artery. Straight aneurysm clips usually fit well for type 1 and type 2 projections (Fig. 6.4). As mentioned before, we recommend temporary clipping of the ipsilateral A1 whenever the aneurysm neck is wider than the A1 diameter.
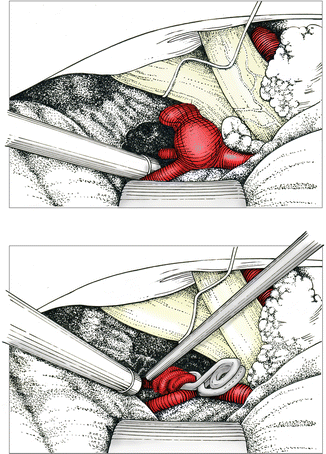
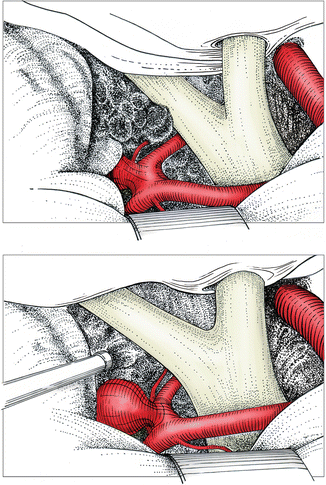
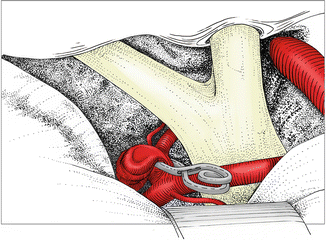

Fig. 6.2
Dissecting the aneurysm and identifying both A2 segments. The white material on the right of the chiasm is a gelatin pledget. Clipping and verification of adequate flow in both A2s by means of the micro-Doppler probe

Fig. 6.3
Anteriorly projecting anterior communicating aneurysm (type 2) approached from the right. Surgical field following careful removal of cisternal clot

Fig. 6.4
Clipping with a straight clip parallel to the anterior communicating artery
Following application of the pilot clip, the situs is now closely inspected. Both branches are checked to see that the aneurysm neck is completely obliterated and a cerebral artery has not been trapped. Now the contralateral A2 origin must be identified. If the clip has grasped the contralateral A2, a second parallel clip should be applied a bit higher on the aneurysm neck. The first clip can then be corrected, possibly after additional dissection of the A2 origin and separation from the neck. Anatomic variations of the A2 segments must be kept in mind at this stage. The most common error is to ignore the situation in which there are three A2 segments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








