, Nima Etminan1 and Daniel Hänggi1, 2
(1)
Neurochirurgische Klinik, Universitätsklinikum Düsseldorf, Düsseldorf, Germany
(2)
Medical Art Christine Opfermann-Rüngeler, Zentrum für Anatomie Heinrich Heine Universität, Düsseldorf, Germany
Aneurysms of the internal carotid artery (ICA) are subdivided according to their location along the ICA into proximal or paraclinoid aneurysms, aneurysms of the middle segment, and terminal aneurysms. Aneurysms may be related to branches of the ICA—that is, the ophthalmic artery, the posterior communicating artery (Pcom), or the anterior choroidal artery (AChA)—but a considerable proportion are unrelated to side branches. Aneurysms of the ICA can reach giant size and present with optic nerve compression.
Very small, blister-like aneurysms that present with subarachnoid hemorrhage (SAH) typically also occur on the ICA. Although surgical management using wrapping techniques is possible, the trend in the literature indicates a clear shift toward endovascular management of blister-like ICA aneurysms using stents or flow diverters, so these aneurysms are not considered in this chapter.
Saccular ICA aneurysms presenting with SAH should be treated by the endovascular route if that is considered feasible, in light of the neck configuration. The best treatment for large aneurysms leading to optic nerve compression, or third nerve palsy in the case of the ICA-Pcom aneurysm, is a matter of debate. Microsurgical clipping leads to immediate relief of the compression, thus creating optimal conditions for recovery. On the other hand, a significant rate of recovery from both vision loss and third nerve palsy is reported following endovascular treatment. Nevertheless, the use of endovascular tools to manage large and giant aneurysms is still disappointing with regard to early complication rates and long-term stability. At this time, we recommend microsurgical obliteration as first choice, if the specific anatomy of the aneurysm appears favorable for exposure and clip elimination.
8.1 ICA–Ophthalmic Artery Aneurysms
8.1.1 General Considerations
ICA–ophthalmic artery aneurysms are the most frequent and clinically most important subtype of the paraclinoid aneurysms. They are located dorsally on the ICA and project toward the optic nerve. They present either with unilateral visual loss or with SAH. Aneurysms presenting with optic nerve compression usually have considerable size, which translates to specific challenges for the microsurgical approach.
The ophthalmic artery originates from the intradural segment of the ICA in over 90 % of individuals. Nonetheless, complete visualization of the base of the aneurysm and in particular of the ophthalmic artery requires extensive dissection with removal of the anterior clinoid process. Because extensive bone removal is associated with additional risk, we do not recommend its general and uncritical use. Some ICA–ophthalmic artery aneurysms are partially located in the cavernous sinus. Postoperative neck remnants in the cavernous sinus usually can be treated well via the endovascular route, and it appears not to be justified to extend intraoperative dissection into the cavernous sinus.
ICA–ophthalmic artery aneurysms present a unique challenge: proximal control is not available during dissection and clipping. Proximal control is important not only to control bleeding in case of intraoperative aneurysm rupture but also to reduce intra-aneurysmal pressure during clip application. Reducing intra-aneurysmal pressure during clip application is essential with large ICA–ophthalmic artery aneurysms; otherwise, they will tear at the base when the clip is applied.
An obvious solution could be to control the extracranial internal carotid artery at the neck by a separate surgical exposure or by the endovascular route with the help of a balloon catheter; but blocking the proximal ICA alone does not help much, as retrograde flow through the ophthalmic artery cannot be controlled in this way. The arterial collateralization of the face is so rich that occlusion of the cervical external carotid artery is useless. Batjer and colleagues [1] developed the so-called retrograde suction decompression technique to cope with the problem (compare Fig. 8.1). Through a catheter inserted in the cervical carotid artery, blood is aspirated during clip application. Today this can be achieved effectively via the endovascular route if intraoperative angiography or a hybrid operating suite is available.
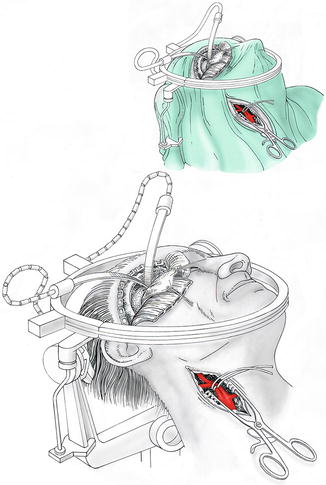

Fig. 8.1
Simultaneous craniotomy and exposure of the cervical carotid bifurcation for complex paraophthalmic aneurysms (OR setting). Details of the simultaneous approach to cranial and cervical exposure for complex paraophthalmic aneurysms
Alternatives to the suction decompression method to render the aneurysm supple during clip application include temporary deep hypotension or short-term cardiac arrest using adenosine [2, 3]. These methods are simpler than retrograde suction decompression and complications are rare if they are used correctly (mainly by limiting the duration to a few minutes). Normotensive and otherwise healthy patients tolerate well induced hypotension to a mean arterial pressure of 40 mmHg for a maximum of 5 min. We recommend the use of induced hypotension if the aneurysm has a clearly delineated neck with preservation of the contour of the parent artery; we reserve retrograde suction decompression for broad-based aneurysms with inclusion of the ICA wall.
8.1.2 Dissection of ICA–Ophthalmic Artery Aneurysm and Clipping
A custom-tailored pterional craniotomy as described in detail in Chap. 4 (Fig. 8.2) is used for the approach. The head is positioned with an approximately 45-degree rotation to the contralateral side and slightly hyperextended. A spinal drain is inserted in case of recent SAH, unless ventriculostomy has been placed on the basis of a World Federation of Neurosurgical Societies (WFNS) grade of 4 or worse.
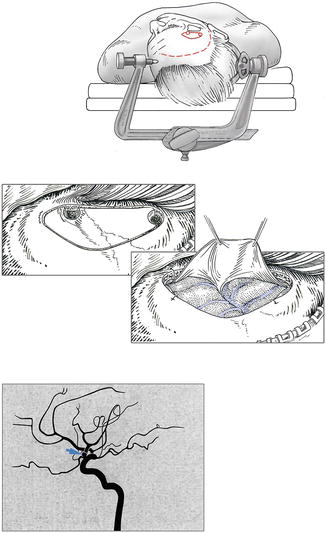

Fig. 8.2
Custom-tailored pterional craniotomy: head positioning, skin incision (broken line), craniotomy (continuous line). Dural opening. Digital subtraction angiography showing aneurysm (arrow)
Before opening the dura, sufficient brain relaxation is achieved by drainage of 50–100 mL of CSF, in case of SAH, and by mannitol if necessary (1–2 g/kg body weight). Because a larger aneurysm requires a large working space, the Sylvian fissure should be completely opened from the periphery down to the clinoid, to allow mobilization of the frontal and orbital cortex without applying undue pressure with a retractor blade. Care needs to be taken not to tear the aneurysm when opening the carotid cistern. For smaller aneurysms, splitting the fissure may be limited to the central aspect. Following gentle elevation of the orbital cortex, the proximal carotid artery and the optic nerve are identified. The optic nerve and the carotid artery are freed of arachnoid adhesions and the prechiasmatic cistern is opened. The distal delineation of the aneurysm neck comes in view at this point. Further dissection of the aneurysm neck clarifies whether removal of the anterior clinoid process becomes necessary. If the dissector can be passed proximally underneath the clinoid over the carotid, removal is not necessary. Otherwise, the anterior clinoid is gently drilled with a diamond drill and the optic nerve is unroofed by splitting the falciform ligament. This maneuver provides some additional space and allows visualization of the ICA down to the entry point into the cavernous sinus. Dissection of the aneurysm neck is completed now on the proximal side beneath the clinoid and the optic foramen. Because the aneurysm obscures vision, the back side usually cannot be seen completely. Dissection is complete when the tip of the dissector reaches bone.
At that time, decisions must be made with regard to making the aneurysm supple for clip application and with regard to the clip technique. If the neck is relatively narrow, short-term induced hypotension is appropriate, and a straight clip can be passed over the top of the ICA. As an alternative, sequential clipping with a palisade of small clips can be used (Fig. 8.3).
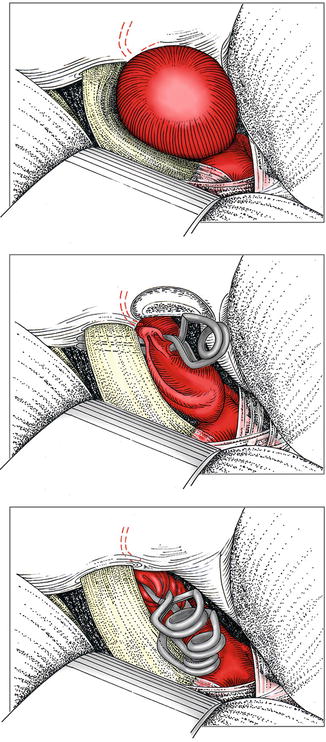

Fig. 8.3
Paraophthalmic aneurysm showing compression of the optic nerve and the presumed course of the ophthalmic artery. Horizontal clip positioning (variant a). Stepwise vertical clipping with multiple clips (variant b)
ICA–ophthalmic artery aneurysms differ from other aneurysms also with regard to temporary clipping if proximal control can be obtained beneath the clinoid. If a sufficient segment of proximal ICA is available, proximal temporary clipping should be done. The effect of proximal ICA clipping on the intra-aneurysmal pressure varies considerably, however, owing to retrograde flow from the distal ICA and the ophthalmic artery. The usual response to insufficient aneurysm relaxation following clipping of the proximal parent artery is additional distal clipping to block retrograde flow, but in the case of an ICA–ophthalmic artery aneurysm, the usual result of distal clipping is an increase in intra-aneurysmal pressure because residual inflow comes from the ophthalmic artery, and applying a temporary clip on the distal ICA blocks the outflow and raises the intra-aneurysmal pressure again to systemic arterial pressure. Therefore, the effect of proximal temporary clipping on intra-aneurysmal turgor must be watched carefully. If it is unsatisfactory, improvement can be expected only from systemic hypotension or other measures as discussed above.
8.1.3 Special Aspects
The retrograde suction decompression method, open or via the endovascular route, is generally accepted for giant and broad-based aneurysms. Although this maneuver is definitely helpful, it is by no means the cure for all problems and is associated with its own specific complications, such as thromboembolism. We prefer systemic hypotension, except with giant aneurysms. We developed a special balloon-assisted method (further discussed in Chap. 10) to temporarily occlude the aneurysm neck, but experience to date is not yet sufficient for a final assessment of its benefits and risks.
8.2 ICA–Posterior Communicating Artery Aneurysms
8.2.1 General Considerations
Today, patients with Pcom aneurysms are considered candidates primarily for microsurgery if they have hematomas, recurrent aneurysms after coiling, Pcom arising from the aneurysm neck, aneurysms causing oculomotor nerve palsy, or multiple aneurysms.
In principle, aneurysms at the ICA-Pcom position are straightforward. Exposure does not provide major challenges, as the approach path is naturally quite wide. There is no need for skull base drilling or significant brain retraction. Proximal control is easy to obtain, and clip application is not critical, as there are no critical perforators in the immediate vicinity. These initial impressions are counterbalanced, however, by their relatively proximal location on the vascular tree. If the parent artery cannot be preserved, ischemic damage is often major, depending on the collateral flow. In the worst case, hemispheric infarction can result from failed surgery.
A few points require particular attention, and some pitfalls must be avoided. The Pcom occasionally has a very proximal origin, with resulting difficulty in obtaining proximal control. It may be necessary in some cases to drill the anterior clinoid process in order to expose the proximal ICA. A further difficulty arises from broad-necked aneurysms that incorporate the origin of the Pcom. This artery should be preserved by all means; preservation is critical when the Pcom has a large diameter—that is, when it is supplying the posterior cerebral artery. In the case of a large Pcom, the hypothalamic and mesencephalic perforators arising from this artery originate on the ICA side of the Pcom, and occlusion of the Pcom origin at the ICA also leads to occlusion or thrombosis of these critical perforators, with subsequent hemiparesis.
A further particularity of the ICA-Pcom aneurysm relates to the age distribution of the patients. On one hand, ICA-Pcom aneurysms represent a preferential location in young patients. It appears that in young patients, these aneurysms have a relatively high rate of recurrence if not perfectly eliminated. Therefore it is important to strive for a perfect solution, using a curved clip to optimally match the curvature of the ICA. On the other hand, the ICA-Pcom location is also a preferential aneurysm site in elderly women. In this group particularly, the aneurysm neck is often wide, and the Pcom occasionally arises from the neck. In this situation, it is often necessary to leave a small part of the aneurysm open in the form of an infundibulum for the Pcom. This is achieved best by using a straight clip that imperfectly matches the curvature of the carotid wall. In our experience, this residual dog-ear does not translate to the risk of aneurysm recurrence in elderly patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








