Group
Brain markers
Brain structure
Brain genes
Behavior
nNOS
Reelin
GFAP
Pyramidal cell atrophy
Brain atrophy
MBP
PLP
Net-nd P
HSCP70
Prepulse inhibition
Influenza mouse model
Adulthood
↓
↓
↑
+
+
↓
↓
↑
↑
Abnormal
Schizophrenia
Adulthood
↓
↓
↓/−
+
+
↓
↓
↑
↑
Abnormal
A further promising animal model is the administration of viral mimetic polyriboinosinic–polyribocytidilic acid (PolyI:C) in vulnerable periods during pregnancy: Descendants of female mice, who were exposed to PolyI:C at day 9, show changes in brain morphology, physiology, and chemistry as well as in behavior after puberty that are partly reminiscent to changes observed in human schizophrenia (e.g., Meyer and Feldon 2010; Winter et al. 2009). Especially, the following findings were described (Meyer 2014):
Impairments in several behavioral measurements incl. PPI, LI, startle response, sensory gating etc.
Changes of interleukin-10 and -1 beta, and other immune reactions
Changes of GABA-A receptors in limbic areas
Dopamine- and glutamate related pharmacological and neuroanatomical
Disturbances
Reduced D1 receptors in PFC and reduced hippocampal NMDA receptor
Increased number of mesencephalic dopamine neurons in the fetal brain
(middle/late gestation) (accompanied by specific gene changes)
Reduction of Reelin- and parvalbumin expressing PFC neurons
Evidence has shown that the time of prenatal insult may provide distinct changes in the exposed offspring. In a recent series of experiments by Meyer et al. (2006) using the viral mimic polyribocytidilic acid (PolyI:C) at E9 (which corresponds to midpregnancy) and E17 (which corresponds to late pregnancy) there were distinct behavioral deficits, neuropathological differences, and acute cytokine responses (Meyer et al. 2006). Adult mice that were exposed on E9 displayed reduced exploratory behavior while those exposed on E17 displayed perseverative behavior (Meyer et al. 2006). At P24, mice that were exposed on E9 displayed a more pronounced reduction of Reelin immunoreactivity in hippocampus than mice exposed at E17 (Meyer et al. 2006). In contrast, mice exposed at E17 displayed an increase in apoptosis as visualized by immunoreactivity of caspase-3, a key enzyme involved in apoptosis (Rami 2003), in the dorsal dentate gyrus (Meyer et al. 2006). Finally, Meyer et al. (2006) found that late gestational immune challenge uniquely stimulated increased IL-10 and TNF-α in fetal brain (Meyer et al. 2006). Taken together, these results provide evidence that the time of prenatal insult results in important differences that are persistent through adulthood.
Neurochemical Findings in the Influenza and PolyI:C Models
Since neurochemical alterations such as in the dopaminergic, glutamatergic, or serotonergic system are highly characteristic for the neurobiology of schizophrenia, some of such findings in the animal models should be reported here in greater detail. The efficacy of dopamine D2 receptor blocking drugs in the treatment of schizophrenia, as well as SPECT studies on neuroleptic naïve patients, suggests that dopamine hyperfunction in the ventral striatum and dopamine hypofunction in the prefrontal cortex may be responsible for the positive symptomology of schizophrenia (Abi-Dargham et al. 2000; Abi-Dargham 2002). Additionally, electrophysiological studies have suggested increased serotonergic function in schizophrenia (Juckel et al. 2003a, b, 2008).
As mentioned above, viral infection causes deleterious effects on brain structure and function in mouse offspring following late first trimester (E9) and late second trimester (E18) administration of influenza virus. Neurochemical analysis following infection on E18 using this model has revealed significantly altered levels of serotonin, 5-hydroxyindoleacetic acid, taurine, but not dopamine. In order to monitor these different patterns of monoamine expression in exposed offspring in more detail and to see if there are changes in the dopamine system at another time point, C57BL6J mice were infected with a sublethal dose of human influenza virus or sham-infected using vehicle solution on E16. Male offspring of the infected mice were collected at P0, P14, and P56, their brains removed and cerebellum dissected and flash frozen. Dopamine and serotonin levels were then measured using HPLC-ED technique. When compared to controls, there was a significant decrease in serotonin levels in the cerebella of virally exposed mice at P14. No differences in levels of dopamine were observed in exposed and control mice although there was a significant decrease in dopamine at P14 and P56 when compared to P0. This study (Winter et al. 2008) has shown that the serotonergic system is disrupted following prenatal viral infection potentially modelling disruptions that occur in patients with schizophrenia, but there was no significant effect on the dopaminergic system. In an additional study (Fatemi et al. 2008), microarray, qRT-PCR, DTI and MRI scanning, western blotting and neurochemical analysis were performed to detect differences in gene expression and brain atrophy. Expression of several genes associated with schizophrenia or autism including Sema3a, Trfr2 and Vldlr was found to be altered as were protein levels of Foxp2. E18 infection of C57BL6J mice with a sublethal dose of human influenza virus led to significant gene alterations in frontal, hippocampal, and cerebellar cortices of developing mouse progeny. Brain imaging revealed significant atrophy in several brain areas and white matter thinning in corpus callosum. Finally, neurochemical analysis revealed significantly altered levels of serotonin (P14, P35), 5-hydroxyindoleacetic acid (P14), and taurine (P35), but again not concerning the dopaminergic or the glutamatergic system.
In the established mouse model mimicked viral-like infection with PolyI:C, we were able to show much clearer neurochemical effects in one study (Winter et al. 2008) as in the influenza model. Prenatal infection-induced changes in brain and behavioral functions are found to be associated with multiple changes at the neurochemical level. Pregnant dams on gestation day 9 were exposed to viral mimetic polyriboinosinic–polyribocytidilic acid (PolyI:C, 5 mg/kg i.v.) or vehicle treatment, and basal neurotransmitter levels were then compared in the adult brains of animals born to PolyI:C- or vehicle-treated mothers by high-performance liquid chromatography on post-mortem tissue. We found that prenatal immune activation significantly increased the levels of dopamine and its major metabolites in the lateral globus pallidus and prefrontal cortex, whilst at the same time it decreased serotonin and its metabolite in the hippocampus, nucleus accumbens, and lateral globus pallidus. In addition, a specific reduction of the inhibitory amino acid taurine in the hippocampus was noted in prenatally PolyI:C-exposed offspring relative to controls, whereas central glutamate and c-aminobutyric acid (GABA) content was largely unaffected by prenatal immune activation. These results thus confirm that maternal immunological stimulation during early/middle pregnancy is sufficient to induce long-term changes in multiple neurotransmitter levels in the brains of adult offspring. This further supports the possibility that infection-mediated interference with early fetal brain development may predispose the developing organism to the emergence of neurochemical imbalances in adulthood, which may be critically involved in the precipitation of adult behavioral and pharmacological abnormalities after prenatal immune challenge (Fig. 1.1, Table 1.2).
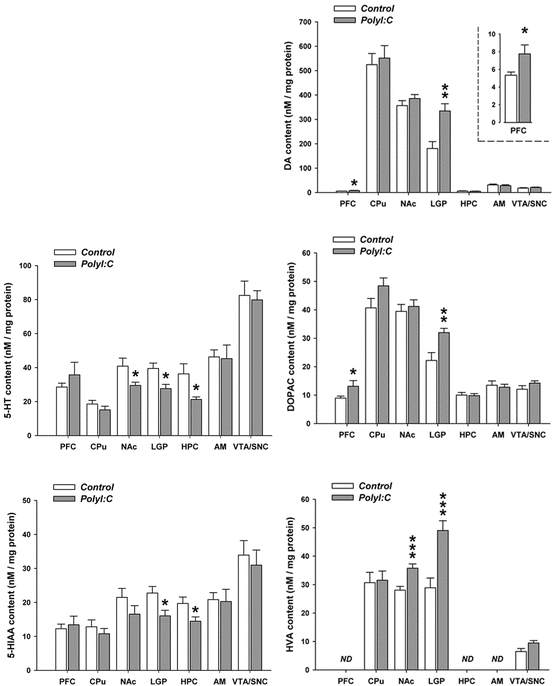

Fig. 1.1
PolyI:C administration at day E9 and measurement of neurotransmitters at day P84 (from Winter et al. 2008)
Table 1.2
PolyI:C administration at day E9 and measurement of neurotransmitters at day P84 (from Winter et al. 2008)
GABA | GLU | GABA | GLU | |
|---|---|---|---|---|
NaCl | PolyI:C | |||
PFC | 11.82 ± 1.24 | 89.33 ± 8.28 | 12.83 ± 1.12 | 88.53 ± 8.72 |
CPu | 11.84 ± 1.13 | 70.05 ± 6.85 | 10.83 ± 0.86 | 64.65 ± 5.64 |
NAc | 29.20 ± 3.14 | 60.53 ± 5.14 | 24.52 ± 2.64 | 61.82 ± 6.15 |
LGP | 27.93 ± 3.36 | 52.43 ± 6.82 | 32.04 ± 4.12 | 52.85 ± 4.97 |
HPC | 14.54 ± 2.47 | 64.82 ± 11.31 | 14.82 ± 1.45 | 64.54 ± 6.35 |
AM | 26.51 ± 2.99 | 78.04 ± 10.14 | 25.22 ± 2.75 | 69.52 ± 10.21 |
VTA/SN | 39.70 ± 6.03 | 46.43 ± 6.72 | 35.97 ± 4.77 | 41.21 ± 7.15 |
Microglial Activation
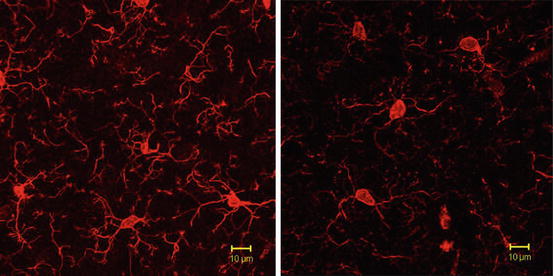
According to the hypothesis above declared, a first own study (Juckel et al. 2011) was aimed to explore microglial activation in PolyI:C exposed mice at postnatal day 30 quantitatively and qualitatively in comparison with NaCl exposed control animals in brain regions involved in the pathogenesis of schizophrenia (frontal cortex, hippocampus, and striatum). Female and male BALB/c mice were maintained under standard laboratory conditions by breeding. Mice were mated overnight, and the presence of vaginal plug marked that day as embryonic day 0. At embryonic day (ED) 9 pregnant mice were treated with intraperitoneal injections of PolyI:C at a single dose of 20 mg/kg. Control mice were given injections of sterile sodium chloride (0.9 %). After this procedure pregnant mice were single housed. Thirty days after birth, descendants were deeply anesthetized and transcardially perfused. Subsequently, 40-μm-thick sagittal sections were cut by using a vibratome. Fluorescence immunostaining was performed according to standard protocols (e.g., Ohsawa et al. 2000). Brain sections were incubated with rabbit polyclonal anti-Iba1 antibody. In addition, a combined CD11b/Iba1 staining was performed to reveal the activation and immune competence of microglial cells. Immunostaining was performed in offspring from PolyI:C treated mice and respective controls. Averaged values from two sagittal sections were taken at the medio-lateral position. Quantitative evaluation of Iba1-positive microglia cells was done in four brain areas: hippocampus, frontal cortex, striatum, and as a control region the occipital cortex. Furthermore, microglial branches and processes were analyzed by using a confocal microscope. PolyI:C treatment caused a significant increase in hippocampus and a significant increase in striatum. There were no significant effects of treatment in the frontal cortex as well as in the control region of the occipital cortex. The density of the Iba1-positive processes of the microglial cells was reduced in the brains of the descendants of the injected mothers as compared to the controls. In the control mice, Iba1-positive cells showed round or oval cell bodies with highly branched processes that formed a dense meshwork. In descendants of the mothers injected with PolyI:C, fine branches were largely diminished, particularly in hippocampus. Microglial cells of descendants from PolyI:C infected mothers exhibited significantly less branches and processes as compared to the control mice, which was supported by confocal microscopy showing reduced surface of processes in PolyI:C mice (Fig. 1.2), suggesting that PolyI:C treatment of mothers caused a higher activation status in the offspring generation. Activated and immune competent status of microglial cells could be also demonstrated by combined CD11b/Iba1 staining. This study was able to demonstrate a higher number of activated microglia in the hippocampus and striatum of PolyI:C exposed mice descendants at day 30 which is comparable to adolescence/early adulthood in man, a time window, in which first symptoms of schizophrenia appear or its first manifestation can be seen. The branches and processes of the microglial cells derived from PolyI:C exposed mice were different to those of the control group. While microglial cells from control animals showed more highly branched arborization, indicative for a non-inflammatory state of microglia, while offspring from PolyI:C treated mice were characterized by not or less branched cells, characteristic for an activated and inflammatory or phagocytic state of microglia (e.g., Shapiro et al. 2009; Stence et al. 2001). Activated microglia could lead to neuropil reduction with rarification of synapses, dendrites, and spines in patients with schizophrenic disorders due to a direct neurotoxic effect (e.g., NO, release of cytokines or free radicals) causing the well-known neuroanatomical changes in the early phases of this disease (e.g., Stehen et al. 2006; Witthaus et al. 2009) as well as the psychopathological symptoms and cognitive symptoms. Alternatively, microglial derived mediators could led to neurophysiological changes that cause changes in the neuronal morphology. Given the duration of this activation, one may also consider negative effects on neural stem cell proliferation and integration into the respective brain areas.