Validity
Description
Challenges
Face
The animal model should recapitulate one or more of the core symptoms of the disease
Difficult to replicate the features of suicide in a non-human species
Construct
The model should involve similar neurobiological mechanisms as the human condition
No clearly defined neurobiology or genetics
No well characterised biomarkers
Predictive
The effects of the drug in humans should be accurately predicted by the animal model
Most models have been validated using conventional antidepressants drugs associated with common sites of action
Psychiatric conditions are a challenging area to study using animals as their symptoms are difficult to replicate in a non-human species particularly in the most commonly used laboratory animals, rodents (for discussion see Cryan and Holmes 2005; Nestler and Hyman 2010; Hendrie et al. 2013). For most psychiatric disorders, questionnaire-based measures and/or clinical interviews are the most commonly used diagnostic approach for humans (DSM-V 2013) and translational methods in non-human species are therefore not feasible. SIB is characterised by the emergence of suicidal ideation, attempted or completed suicides which can be attributed to the taking of a therapeutic medication. Quantification of these behaviours also depends on questionnaire-based measures and current FDA guidelines recommend using the Columbia-Suicide Severity Rating Scale (C-SSRS) (Pumariega et al. 2011; Posner et al. 2007; 2011) and the Suicidal Behaviours Questionnaire-Revised (SBQ-R) (Osman et al. 2001) for assessment of SIB at screening and/or during clinical trials. Although a specific animal model or test for SIB has not been developed and validated to date, researchers have looked to the closely related psychiatric disorder, MDD as well as behavioural traits associated with SIB including hopelessness, impulsivity and aggression (Cryan and Holmes 2005; Cryan and Slattery 2007; McArthur and Borsini 2006; Malkesman et al. 2009; Preti 2011).
18.3 Animal Models of Depression
Animal models developed to study MDD have been around since the 1960s when behavioural tests which could predict antidepressant efficacy in man were described (Reserpine model, Askew 1963); forced swim test (FST), Porsolt et al. 1977). These assays went on to form the basis for the successful development of all the modern antidepressant drugs including the serotonin-specific re-uptake inhibitors (SSRIs). Animal models for depression form a logical starting point for investigating SIB in non-human species. MDD is associated with an increased risk of suicide with approximately 90 % of suicide attempts associated with a serious mental health condition (Beautrais et al. 1996). Suicidal ideation is also a common feature of MDD and is one of the symptoms included in the DSM-V diagnostic manual (DSM-V 2013). Treatment-induced depressive symptoms are also seen with a number of drug-treatments which have been associated with SIB and therefore may be a major contributing factor (Nathan et al. 2011; Magin et al. 2005; Bremner and McCaffery 2008; Ahmed et al. 2013). An increased risk of SIB linked to treatment-induced depression during interferon-alpha treatment for hepatitis C has also been reported adding to the argument that pro-depressant effects may be an important contributing factor in the development of SIB (Sockalingam et al. 2011). Although not all patients with symptoms of depression go on to attempt suicide, many of those who do commit suicide have a prior history of a mood disorder, either unipolar or bipolar depression (Beautrais et al. 1996).
Although animal models for depression have been used extensively in basic research, their validity has been increasingly questioned and concerns have been raised about how reliable they are in terms of predicting effects in people. These limitations have been discussed in detail in other articles (Willner 1984, 2005; Nestler et al. 2002; Cryan and Holmes 2005; Cryan and Slattery 2007; Nestler and Hyman 2010; Pollak et al. 2010; Berton et al. 2012 reviews) and are therefore only briefly summarised here to put the subsequent discussions about SIB-related studies using these approaches into context.
When considering animal models of depression, the term ‘model’ is often used to describe both methods to induce a depression-like phenotype and those methods used to assay depression-like behaviour. Stress-related paradigms such as chronic mild stress, early life adversity and psychosocial stress have been shown to induce neurobiological and behavioural deficits thought to reflect a depression-like phenotype (Willner et al 1987, 1992; Willner 1997, 2005; Kudryavtseva et al. 1991; Rygula et al. 2005; Mathews et al. 1996; reviwed by Schmidt et al. 2011). Another approach has been to use olfactory bulbectomy to induce depression in rodents (Leonard 1984; Kelly et al. 1997). Although of interest in terms of the roles these models have played in our understanding of the factors which contribute to MDD, these methods largely depend on assays of depression-related behaviour to validate the generated phenotype. Thus, it is these assays of depression-related behaviour which may provide the most suitable predictive assays for evaluation of SIB in drug development. For the majority of non-human studies into depression, the assays used to quantify mood-related symptoms are designed to provide a measure of either behavioural despair e.g. the FST and tail suspension test (TST), or anhedonia e.g. sucrose preference test (SPT) and intracerebral self stimulation (ICSS) (FST: Porsolt et al. 1977; Detke et al. 1995;TST: Steru et al. 1985; reviewed by Cryan et al. 2005a, b; SPT: Willner et al. 1987; Vogel et al. 1986; ICSS: Zacharko and Anisman 1991). A number of more general behavioural symptoms can also be measured in rodents which may relate to depression e.g. poor coat condition, body weight changes and aggression (Cryan and Holmes 2005; Cryan and Slattery 2007; Hendrie et al. 2013).
18.3.1 Behavioural Despair
Assays of behavioural despair were initially developed and validated using prototypical antidepressant drugs (Porsolt et al. 1977) and are now generally considered to provide a valid approach to predicting antidepressant efficacy for drugs acting through monoaminergic systems, but have limited validity for non-monoaminergic targets (Berton and Nestler 2006). In the classical version of the FST, animals are placed into a cylinder of water from which they can’t escape. When the procedure is repeated 24hrs later, and the immobility time of the animals is measured to provide an indication of behavioural despair. Pre-treatment with antidepressant-like compounds reduces immobility time in this assay (Porsolt et al. 1977). The assay has subsequently been adapted for mice where the water is replaced with suspension by the tail (Steru et al. 1985). In fact, the first assay used to predict antidepressant-like effects was reserpine-induced behaviours where the monoamine-depleting effects of reserpine treatment were attenuated by imipramine-like compounds (Askew 1963). The FST and TST have shown good predictive validity in terms of antidepressant activity for drugs acting through monoaminergic targets but these assays have also been heavily criticised (Porsolt et al. 1977; Steru et al. 1985; Slattery and Cryan 2012; for reviews see Cryan et al. 2005a, b; Berton and Nestler 2006). The potential issues with these assays may stem from their initial development, which was based on validation using conventional antidepressant drugs such as the tricyclic antidepressants (TCA) and monoamine oxidase inhibitors (MAOI) (Porsolt et al. 1977). The mismatch between the acute time course of effects in the rodent assays and delayed clinical benefits seen in patients following antidepressant drug treatment has also been seen as a limitation of these models. One non-monaminergic antidepressant, ketamine, an NMDA receptor antagonist and rapidly acting antidepressant, acts to reduce immobility time in the FST (e.g. Garcia et al. 2009), however issues relating to the locomotor effects of ketamine are a potential confound (Slattery and Cryan 2012). Locomotor effects in this stress-related paradigm are not only an issue with ketamine, and the original validation data presented for the assay included the caveat that locomotor effects were a potential non-specific confound (Porsolt et al. 1977; Slattery and Cryan 2012). Many researchers now conclude that the FST and TST provide valid models for predicting antidepressant efficacy for monoaminergic drugs and ‘me-to’ compounds, but are limited in their sensitivity to drugs acting through novel mechanisms such as those associated with SIB. For example rimonabant, a cannabinoid1-receptor antagonist/inverse agonist developed to treat obesity had been shown to be an antidepressant in the FST (Griebel et al. 2005) but was subsequently withdrawn from the European market in 2009 after it was found to induce depression, anxiety and suicidal ideation in obese patients (Topol et al. 2010). As summarised in Table 18.2, studies investigating the effects of pro-depressant drugs using behavioural despair methods such as the FST and TST have failed to provide reliable predictive data. Rimonabant was shown to exhibit an antidepressant or neutral profile following acute administration although chronic treatments have been reported to increase immobility time indicative of a pro-depressant effect (Table 18.2). Other pro-depressant drugs have also failed to show effects in these assays although studies are still somewhat limited (see Table 18.2 for details). Overall, the behavioural despair methods appear to lack sensitivity to most pro-depressant drugs following acute administration (Table 18.2). Using a chronic treatment may be a better approach and some evidence for predictive validity has been shown, although this may be restricted to drugs from certain classes. For example, no effects or an antidepressant profile were shown following acute or chronic administration of anti-epileptics drugs such as lithium (Table 18.2). As discussed earlier, one potential issue with the FST and TST may be due to its sensitivity to drugs acting predominantly through monoaminergic pathways (Berton and Nestler 2006). Other sites of action may be associated with pro-depressant effects which are not evident following either acute or chronic treatments in the FST or TST.
Table 18.2
Effects of drugs associated with SIB in animal models of depression (behavioural despair and anhedonia)
Assay | FST/TST | SPT | ICSS | References |
|---|---|---|---|---|
Rimonabant | Acute—no effect or ↓ immobility Chronic—↑ immobility | Acute—no effect Chronic—↓ preference | Acute—↑ threshold or no effect Chronic—no info | Adamczyk et al. (2008) Griebel et al. (2005) Gamble-George et al. (2013) Rademacher and Hillard (2007) Beyer et al. (2010) Deroche-Gamonet et al. (2001 ) |
Retinoic acid | Acute—no effect Chronic—immobility or no effect | Chronic; no effect | No info | O’Reilly et al. (2006 ) Ferguson et al. (2005) Cai et al. (2010) Trent et al. (2009) |
IFNα | Acute—↑ immobility Chronic—↑ immobility | Acute—↓ preference Chronic—↓ preference | Acute—no effect Acute (LPS)—↑ threshold | Makino et al. (2000) Sammut et al. (2001) Sammut et al. (2002) Kentner et al. (2007) |
Varenicline | Acute—↓ immobility or no effect Chronic—no info | Acute—no info Chronic—no info | Acute—↓ threshold | Caldarone et al. (2011) Turner et al. (2010) Rollema et al. (2009) Igari et al. (2014) |
Valproate | Acute—no effect (LiCl—↓ immobility) Chronic—↓ immobility | Acute—no info Chronic—no info | Acute—no effect (LiCl—↑ threshold) Chronic—No info | Tomasiewicz et al. (2006) Semba et al. (1989) |
Stress | Acute—no effect Chronic—↑ immobility | Acute—↓ preference Chronic—↑ preference | Chronic—↑ threshold | Platt and Stone (1982) Moreau et al. (1992) |
18.3.2 Anhedonia
Anhedonia, the reduced sensitivity or experience of reward, is one of the core symptoms of MDD although patients can be diagnosed with MDD in the absence of anhedonia if they exhibit other symptoms such as low mood (DSM-V 2013). Although anhedonia is also commonly seen in other psychiatric disorders, such as schizophrenia and during withdrawal from drugs of abuse. Therefore, drug-induced anhedonia may represent an important pre-clinical screen for assessing risks associated with SIB.
Anhedonia is perhaps one of the more translatable symptoms of emotional dysfunction which can be measured in rodents (for discussion see Anisman and Matheson 2005). To quantify sensitivity to reward using a rodent model, one of the most widely used assays is ICSS (Zacharko and Anisman 1991). The method was originally developed to investigate reward mechanisms associated with addiction and during withdrawal from drugs of abuse (Phillips et al. 1983; Schaefer and Michael 1983). The procedure involves the implantation of an electrode into the medial forebrain bundle and the animal is trained to associated a lever press response with direct stimulation of the associated dopaminergic pathway (for review see Liebman 1983). To quantify the hedonic response, the threshold for stimulation is determined for each animal with a higher intensity of stimulation needed to maintain responding in animals exhibiting anhedonia. An alternative approach developed in the context of depression research is the SPT test or sucrose consumption test (Willner et al. 1987; Vogel et al. 1986). These assays depend on the animals’ ability to experience reward associated with a weak sucrose solution (usually 1 %). Either total consumption or preference for the 1 % sucrose versus water can provide a measure of anhedonia in rodents (Willner et al. 1987; Vogel et al. 1986).
In terms of depression, there is good evidence for a stress-induced anhedonia in rodents following exposure to chronic mild stress (Willner et al. 1987) or chronic social defeat stress (Rygula et al. 2005). Studies have also shown that the stress-induced anhedonia is sensitive to chronic but not acute antidepressant drug treatment, mirroring clinical observations of the time course of therapeutic benefit (Willner et al. 1987; Vogel et al. 1986; Zacharko and Anisman 1991). Interestingly, treatment with the NMDA antagonist, ketamine, has been associated with a rapid reversal of SIB in patients (Diaz-Granados et al. 2010) although studies in animals using the SPT found effects with ketamine only after chronic administration (Garcia et al. 2009). Anhedonic changes similar to those seen in depression appear to be relatively well replicated in animals although as yet, only limited information is available for drug-induced anhedonia in the context of SIB (see Table 18.2). Studies using either ICSS or the SPT have investigated several different pro-depressant manipulations with the details of their findings summarised in Table 18.2. It is clear that consistency across drugs from different classes and tests of anhedonia has not been achieved although the number of studies reported in the literature is still very limited. Rimonabant has been shown to induce anhedonia following chronic but not acute administration when tested using the SPT (Rademacher and Hillard 2007) but effects measured using ICSS were inconsistent with some reports of an anhedonic effect following acute treatment whilst others report a lack of effect following either acute or chronic treatment (Deroche-Gamonet et al. 2001). Similar to the results for the FST, varenicline appeared to exhibit an antidepressant-like profile and in the ICSS paradigm it reduced the threshold for stimulation (Igari et al. 2014). Other drugs tested in models of anhedonia include retinoic acid, interferon-alpha and the anti-epileptics although their effects are also inconsistent (see Table 18.2). Thus, whilst the translational validity of animal models of anhedonia is high, the results to date do not provide evidence of good predictive validity for SIB.
18.3.3 Cognitive Neuropsychological Models of Depression
In 2003, studies using the antidepressant, reboxetine, found that acute treatments in healthy volunteers led to a positive shift in the processing of emotional information (Harmer et al. 2003). These changes were observed using neuropsychological tests of emotional behaviour and revealed that drug-induced changes in these cognitive processes were present in the absence of any subjective effects on mood (Harmer et al. 2003, see also reviews Harmer et al. 2009b Pringle et al. 2011; Harmer 2013). Cognitive processes in depression have been discussed for many decades with the first proposal of a cognitive theory of depression described in 1967 (Beck 1967, 1976). The more recent developments in computer-based neuropsychological tests have allowed for much more sensitive, objective measures of emotional behaviour to be made in human participants and renewed interest (For discussion see Robinson and Sahakian 2008; Clark et al. 2009; Harmer et al. 2009a; Elliott et al. 2011; Roiser et al. 2012; Roiser and Sahakain 2013). Studies in depressed patients have shown consistent deficits in emotion-related cognition including changes in emotional interpretation, learning and memory, with a shift towards a negative emotional interpretation (see Mathews and MacLeod 2005; Clark et al. 2009; Gotlib and Joormann 2010 for reviews). It is not yet known whether these negative cognitive affective biases (CAB) are a cause or consequence of the disease however, they appear to represent a possible biomarker for depression. This theory is supported by evidence that individuals with a vulnerability to MDD also exhibit similar negative biases without overt disease symptoms (Hayward et al. 2005; Joormann et al. 2007; Chan et al. 2007; Dearing and Gotlib 2009). Perhaps, the most important development from this work has been in the pharmacology of CABs and studies in healthy volunteers which suggest this type of biomarker may also provide an early indicator of both antidepressant efficacy and pro-depressant risk (see review by Pringle et al. 2011).
The majority of studies to date have focussed on whether early changes in CABs predict long-term antidepressant efficacy in patients (see review by Pringle et al. 2011). Using a range of antidepressant drugs from different classes and treatment regimes of single dose or 7 day of treatments, a positive bias in emotional processing, opposite to those seen in depression, has been reported (see review by Pringle et al. 2011). Although not all treatments act in an identical manner, all drugs which are effective antidepressants in humans induce a positive bias in one of more of the emotional processing tests carried out (for review see Pringle et al. 2011). Using a similar battery of neuropsychological tests, acute or short-term administration of the pro-depressant drug, rimonabant was shown to induce negative CABs (Horder et al. 2009, 2012). Interestingly, the partial agonist at the α4β2 subtype of nicotinic acetylcholine receptor, varenicline, did not have any effects following short term treatment (Mocking et al. 2013). As well as the potential to use this type of neuropsychological approach to study pro-depressant risk in humans, methods assaying objective measures of emotional processing also provide the basis for the development of new, translational methods for use in animals.
Although studies in humans use language-based approaches or emotional facial expressions which cannot be directly translated to non-human tasks, the principles which underpin these tests can be translated (Paul et al. 2005). The first study to achieve this was reported by Harding et al. (2004) and described an assay to detect CABs in rodents. The study provided the first evidence that a translational method for studying emotional behaviour in a non-human species was achievable. The first rodent CAB task required animals to learn to associate two distinct auditory cues with different emotionally valenced outcomes: obtaining reward or avoidance of punishment. Once the animals had learnt the associations, CAB was investigated by presenting the animals with intermediate ambiguous tone cues and observing their responses. The ‘optimistic’ rat was predicted to make more responses in anticipation of reward whilst the ‘pessimistic’ rat would make more responses indicating anticipation of punishment. Using a chronic mild stress manipulation, this original study showed that rats in a putative negative affective state were more likely to anticipate negative events similar to what is seen in depressed patients (Wright and Bower 1992). A number of different laboratories have now replicated this work (Enkel et al. 2010; Anderson et al. 2012) and have refined the task design to reduce potential confounds associated with motivational state. There have also been task designs which use spatial cues in place of auditory cues to predict outcomes (for review see Hales et al. 2014). In both the original tone-based operant task and these spatial judgment tasks, a go/no-go task design is used. The potential problem with this design is that any change in motivation for the reward could impact on the ambiguous cue interpretation or the latency to approach the goal pot. In order to see a true judgement bias, go/go presentation of the tone-based operant task using active lever press responses to either obtain reward or avoid punishment has been developed (Enkel et al. 2010; Anderson et al. 2012; Rygula et al. 2012, 2013; Papciak et al. 2013). To date, the numbers of studies using this approach are limited and training protocols indicate that animals require long training periods of up to 3 months to reach a stable level of performance before drug testing can be carried out (for review see Hales et al. 2014). Pharmacological validation of these ambiguous cue interpretation or judgement bias methods is also limited (Anderson et al. 2013; Enkel et al. 2010) and studies investigating pro-depressant drugs have not yet been reported. Studies using the antidepressant fluoxetine have shown a tendency to reduce negative judgement biases following chronic but not acute administration (Anderson et al. 2013). In contrast, the antidepressant reboxetine either alone (Anderson et al. 2013) or in combination with corticosterone (Enkel et al. 2010) induced a negative bias and reduced anticipation of reward. Although the translational potential of this approach appears high, to date, the predictive validity is limited and further studies are needed before the value of this approach for SIB and depression-related studies can be fully evaluated.
An alternative method to study CAB in rodents has also recently been reported (Stuart et al. 2013). This assay uses a similar strategy of ‘reverse translation’ but is based on the memory biases observed in depressed patients as apposed to emotional interpretation biases (see Mathews and MacLeod 2005; Clark et al. 2009; Gotlib and Joormann 2010 for reviews). The rodent affective bias test (ABT) is similar conceptually to the conditioned place preference paradigm (CPP). The basis of the task is the hypothesis that memory for a specific experience is biased by affective state at the time the experience is learnt, which subsequently influences the memory of the relative value of that experience (Stuart et al. 2013). Studies using antidepressant drug treatments from different pharmacological classes suggest that a good correlation between their known clinical efficacy in patients and the drug’s ability to induce a positive bias in the ABT. As well as pharmacological validation, experiments using psychosocial manipulations of affective state also resulted in biases in this assay consistent with their predicted effects on affective state. Exposure to social and environmental enrichment resulted in a positive bias whilst psychosocial stress induced a negative bias (Stuart et al. 2013). Together, these data support the translational and predictive validity of the ABT for depression-related research. In terms of SIB, studies using the ABT have also investigated a number of treatments known to induce depression and/or SIB. The CB1-antagonist, rimonabant and the active ingredient of the anti-acne medication roaccutane, 13-cis-retinoic acid, induced a negative bias in this assay (Stuart et al. 2013, Fig. 18.1). Interestingly, the SSRI antidepressants tend to exhibit a bell-shaped dose response curve in the ABT (Stuart et al. 2013). This observation has not been further investigated but may be relevant to their proposed risks of inducing SIB in vulnerable individuals or at certain doses.
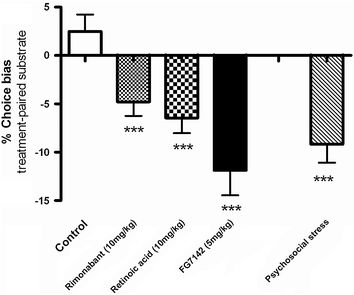

Fig. 18.1
Pharmacological evaluation of the ABT for predicting pro-depressant risk. In the ABT, the percentage choice bias is measured using a preference test where the animal bias towards or away from an experience encountered during antidepressant treatment is quantified. Results are shown the maximal effective dose and a positive control of psychosocial stress. Full dose-response data are given in Stuart et al. 2013. These studies show that three pharmacologically distinct pro-depressant treatments induce similar negative affective biases in the rat ABT. The magnitude of effect is similar to that seen following a stress manipulation (restraint stress and 24 h social isolation). Results are shown for mean ± sem for 16 animals tested using a within-subject affective bias test. *p < 0.05, **p < 0.01, ***p < 0.001 post-hoc one sample t-test versus no bias
Considering all the different depression-related assays which have been used to investigate drugs with known neuropsychiatric risk, pharmacological data for the ABT test shows the greatest level of predictive validity when compared to data obtained from healthy human volunteers and/or patients. The assay has yet to be replicated in other laboratories and further validation data are needed. In particular studies using other drugs with a black box warning for SIB such as the anti-epileptics are needed. The current version of the task is also very labour intensive and would have greater value if it could be modified to an automated version.
18.4 Animal Models of Suicide-Related Behavioural Traits
Although animal models of depression are thought to be a good predictor of SIB and several of the drugs associated with these side effects also induce depression, other behavioural traits associated with suicide are also of potential interest. Suicide arises as a result of multiple behavioural traits, some or all of which may be present. Of specific relevance to SIB are the behavioural traits of hopelessness, impulsivity and aggression (for review see Brezo et al. 2006). Animal models to investigate these behaviours have all been described previously although usually in studies unrelated to SIB. The potential applications for animal models of these suicide-related behavioural traits have been reviewed previously (Malkesman et al. 2009; Preti 2011) and therefore only a brief synopsis is given here. As opposed to a detailed description of the specific methodologies, which are described in detail in Malkesman et al. (2009), particular attention has been made here to the pharmacological and translational validity of these approaches and any studies using drugs associated with SIB (Table 18.3).
Table 18.3




Effects of drugs associated with SIB in animal models of trait behaviours
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





