Fig. 8.1
Anatomical landmarks for the anterior presternocleidomastoid – precarotid approach
Hard palate: atlas arch
Inferior side of the mandible: C1-C2 space
Hyoid bone: C3
Thyroid cartilage: C4-C5 space
Cricoid cartilage: C5-C6 space
Carotid tuberculum (Chassignac’s tuberculum): lateral margin of C6
Other identifiable landmarks are:
The medial margin of the sternocleidomastoid muscle, from the mastoid process to the sternum
The carotid artery , that can be palpated posteriorly and laterally to the medial margin of the sternocleidomastoid muscle.
After the execution of an intraoperative radiography , the skin incision is performed along a line of the neck (transversal if the level to expose is one, oblique along the margin of the sternocleidomastoid muscle if the levels to expose are at least two). The side chosen is generally the left one to reduce the risk of damage to the recurrent laryngeal nerve, because on the right side this nerve has a more variable anatomical route. After the skin incision, superficial haemostasis is performed, the hypodermis is elevated and the platysma is identified. The fascial sheath over the platysma is dissected with the same orientation of the skin incision and then the platysma is separated longitudinally with a smooth dissection, identifying the medial margin of the sternocleidomastoid muscle covered by the deep cervical fascia; the deep cervical fascia is then incised anteriorly to the anterior margin of the sternocleidomastoid muscle. After that, the sternohyoid and sternothyroid muscles are stretched apart medially so that trachea and oesophagus can be dislocated medially, and the carotid sheath, containing the common carotid artery, the jugular vein and the vagus nerve, is identified. Once the carotid artery has been palpated and well identified, the pretracheal fascia is incised starting from the medial side of the sheath and the sheath is stretched apart with the neurovascular bundle of the neck in lateral and external position together with the sternocleidomastoid muscle [12].
With a smooth dissection the cervical vertebrae, covered by the prevertebral fascia, are reached, and the longus colli muscles are identified in both sides of the median line. Laterally to the vertebral bodies are located the cervical sympathetic chain ganglia. With the help of an electrocauterium, the longus colli muscle is sectioned in longitudinal direction, and via subperiostium is dissected together with the anterior longitudinal ligament and stretched apart laterally.
The surgeon identifies the intervertebral disc, places a landmark point with a spinal needle and performs an X-Ray to verify that the disc individuated is the right one.
Autostatic retractors are placed to stretch apart the longus colli muscles and a Caspar distracting system is put in place. The distractor takes advantage of his pins inserted into the vertebral bodies do distract the intervertebral space where the surgeon will be working (Fig. 8.2). The disc is incised with a scalpel and the discectomy is performed from the anterior margin to the posterior one. The fibres of the posterior annulus fibrosus can be detached from the bone, and a cleavage plane with the vertical fibers of the posterior longitudinal ligament can be obtained with the support of a dissector.
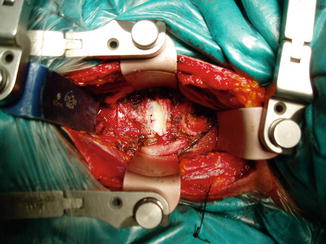

Fig. 8.2
Cervical disk exposed by anterior presternocleidomastoid – precarotid approach
At the end of the procedure, a high speed drill can be used to smooth the marginal osteophytes and to cruentate the endplates until one can obtain a bleeding osseous surface where the implant can be inserted. Once a good visualization of the vertebral canal is obtained, the osteophytes located on the posterior margin can be removed and the opening of the intervertebral foramen can be performed to ultimate the decompression. Once this phase is completed, in the intervertebral space can be placed a bony graft, an interbody cage or a disc prosthesis. At the end of the procedure, after a control X-Ray scan, the operatory field is washed with saline solution, an accurate haemostasis is performed and a drainage is placed. In the final phase the platysma muscle is sutured and the superficial planes are sutured.
Complications
The most frequent complications related to the procedure are: damage to the recurrent laryngeal nerve with palsy of the vocal cords (transitory in 11 % of the cases, permanent in 4 % of the cases), damage of sympathetic nerves or of the stellate ganglion (with Horner’s syndrome), damage of the vascular structures such as carotid artery or internal jugular vein or, less frequently, of the vertebral artery or of the thoracic duct [1, 2]. Furthermore, trachea, esophagus or pharynx can be accidentally perforated during the surgical procedure or as a late complication due to the mobilization of the implants. This kind of approach can be the cause of early damages to the nervous structures. Dysphagia can be an immediate consequence after the surgical intervention in 60 % of the patients or in 5 % of the patients can be evident after 6 months or more. It can derive from anesthesiological irritative factors (usually remains for 24–72 h) or to a difficult orotracheal intubation, damages to the recurrent laryngeal nerve, to the cervical orthosis or to the protrusion, displacement or rejection of the prosthetic material [10, 11]. Among the late consequences there are: displacement of the implant, pseudoarthrosis, adjacent segment syndrome. The incidence of failed bone fusion with pseudoarthrosis and adjacent segment syndrome is variable between 2 and 20 %; it can be defined as the displacement of 2 or more millimetres between two adjacent vertebral bodies (measured at the distal portion of the spinous processes) in the dynamic flexion and extension X-Rays. This clinical condition can be asymptomatic, associated to chronic pain, recurrence of the preoperative symptoms, alterations of the cervical lordosis with local kyphosis or deformity. If asymptomatic the surgical therapy is not indicated. If symptomatic, a further surgical intervention with a new ACDF with the placement of an anterior plaque, a cervical corpectomy with fusion or a posterior cervical fusion when more than three vertebral bodies are involved.
Personal Experiences
Anterior Cervical Discectomy and Fusion ACDF
Our experience has been published on European Spine Journal [13]. It has been the first study to deal with ACDF employing carbon fiber cages with such a long follow-up (77 months, range 54–90 months) and the only one to evaluate the rate of interbody fusion using CT scan . Aim of our study was to demonstrate the high rate of interbody fusion and the low percentile of new adjacent level compression in patients who underwent anterior cervical fusion with carbon fiber cages containing hydroxyapatite. All the patients who had undergone surgical treatment with anterior cervical discectomy and stand-alone cage interbody fusion were included in our study. We used a left anterior retropharyngeal approach. Carbon fiber cages containing hydroxyapatite were implanted in all patients without anterior plating. In our experience, adjacent segment disease was not clinically relevant. We studied our patients by the mean of CT scan with sagittal reconstructed images, that made possible to evaluate the rate of bone fusion. During follow-up, 20 % of the patients included in our study presented AS degeneration, and 10 % of these required a new surgical procedure because of AS disease. The findings evidenced by our experience support the hypothesis of a pathophysiological degeneration of cervical spine by reducing the impact of fusion on adjacent levels. Moreover, the less satisfactory outcome observed in patients over 61 years old, with development of ASD disease, was probably related to the evolution of pathophysiological degeneration of the cervical spine [2].
The ideal cage should correct deformity and provide stability until fusion occurs with no additional morbidity. Carbon fiber cages were introduced almost a decade ago for use as a spacer. They do not induce an inflammatory response, withstand physiological loads and have a modulus of elasticity almost equal to the cortical bone. The Cervical Cages used, are a carbon fiber-reinforced hollow biocompatible polymer implants designed to replace the tricortical bone graft. This cage is radiolucent, which aids in the assessment of bony fusion and have markers to aid in their visualization, radiological assessment, and identification of their position on plain X-ray films. These markers are three intrinsic tantalum beads that serve as radiographic markers. Cervical carbon fiber cages are well documented in their ability to provide structural support while promoting bony fusion. Their radiolucency and biomechanical design properties make them a superior choice among available cages. The potential benefit of enhanced fusion rates and decreased bone donor site morbidity may justify the use of hydroxiapatite with cages. Our experience with carbon fiber cages suggests that these devices represent a valid option for restoring the intervertebral disc space and promoting arthrodesis in cervical disc surgery while their elastic properties minimize the risk of kyphosis, subsidence and adjacent segment disease [2].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








