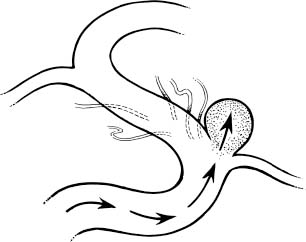
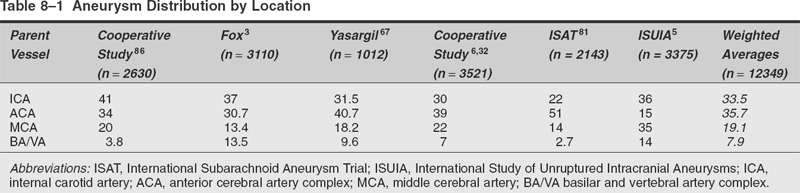
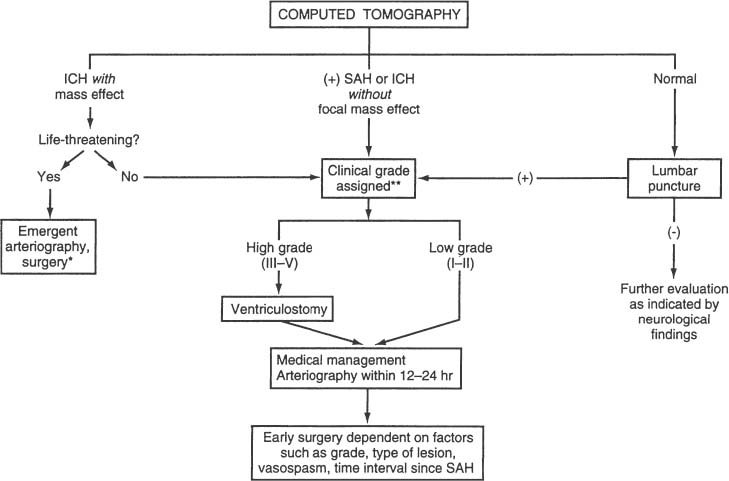
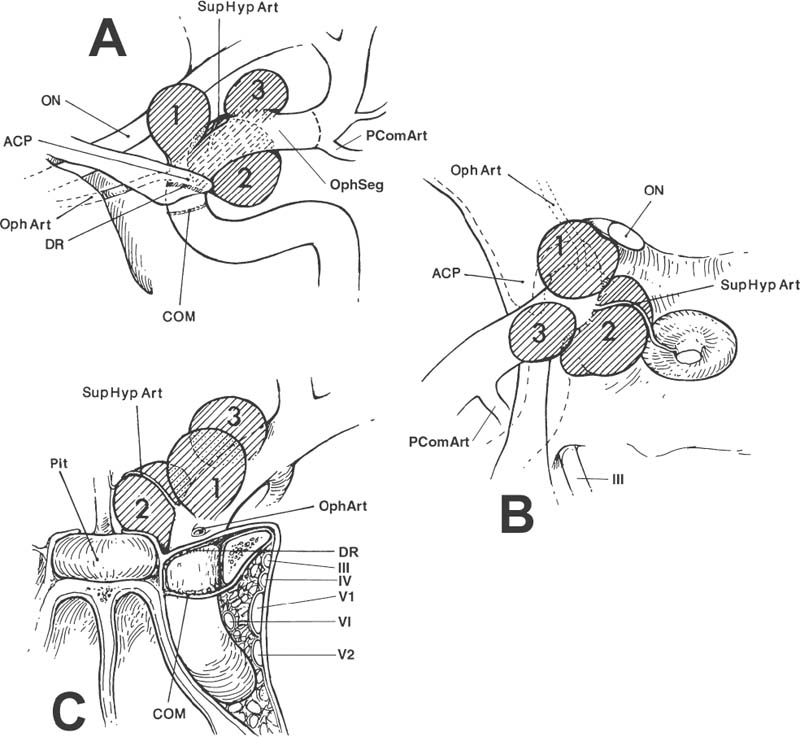
Surgical Treatment of Anterior Circulation Aneurysms Objectives: Upon completion of this chapter, the reader should be able to summarize the microsurgical techniques utilized in the treatment of internal carotid artery, middle cerebral artery, and anterior cerebral artery aneurysms, as well as the results that can be expected with these techniques. Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians. Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity. The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp * The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons. Saccular or berry aneurysms display several anatomic characteristics (i.e., shape, relationship to their artery of origin) that distinguish them from other types of intracranial aneurysms (Fig. 8-1).1,2 Saccular aneurysms typically (1) arise at bifurcations, usually just distal to a branch from a large parent vessel (i.e., internal carotid-posterior communicating [PComArt] junction), (2) arise along a curve of the parent vessel, (3) point in the direction that flow would have proceeded had the curve not been present, (4) are associated with a specific set of perforators, and (5) are best managed surgically with a specific clip type. In this chapter we address the surgical issues associated with saccular aneurysms that arise within the anterior circulation beyond the carotid artery exit from the cavernous sinus. We include internal carotid, anterior cerebral and middle cerebral artery lesions, both ruptured and unruptured. Each patient has a unique set of anatomical and clinical factors that affect the physician’s choice of therapy. The principle anatomical factors that influence treatment of an anterior circulation aneurysm include aneurysm size, location, shape, intraluminal thrombus, and associated calcification. Other clinical factors to be considered include the presence of aneurysm rupture, patient age, general preexisting medical and neurological status, Hunt and Hess grade following subarachnoid hemorrhage (SAH), and other less-quantitative measures that suggest the patient’s ability to tolerate the physiological stress of either open surgical or endovascular treatment. More than 90% of all intracranial aneurysms arise on or near the circle of Willis, the vast majority from the anterior circulation.3 Several large clinical series suggest a slight preponderance of aneurysms on the anterior cerebral artery/anterior communicating artery complex (Table 8-1), followed closely by the internal carotid artery (ICA). Autopsy series (which logically include a higher number of asymptomatic incidentally discovered aneurysms) suggest that middle cerebral artery aneurysms are actually the most common location.3 More recent clinical series suggest that the factors involved in aneurysm formation are different from the factors that promote rupture.4,5 FIGURE 8-1 Intracranial saccular aneurysms generally: (1) arise at a branching site along the parent artery, (2) arise along the outside (convex) surface of a bend in the parent artery, (3) project in the direction of flow (arrows) that the parent artery would have if the bend had not been present, and (4) are associated with a specific set of perforators. Aneurysms at specific sites are usually best obliterated with a specific clip type that conforms to the lesion’s shape, size, and anatomical relationships to the parent vessel, its branches, and adjacent structures. Aneurysm location and rupture risks appear to be influenced by sex and age. Overall, aneurysms are more common in women than men.3,6,7 In children, the male: female ratio at time of presentation is 3:2, in young adults 1:1, and in older adults 2:3.8 In women, the most common aneurysm site, either ruptured or unruptured, is the supraclinoid ICA (66% and 40%, respectively). In men, the most common site for a ruptured aneurysm is the anterior communicating artery complex (44%), but for unruptured aneurysms it is the supraclinoid ICA (34%). Women are much more likely to develop ophthalmic segment (3.3 to 1) or communicating segment (2.1 to 1) aneurysms compared with men, but men are more likely to develop aneurysms of the anterior communicating artery complex (1.4 to 1). Aneurysms can occur at any time in life but are rare in children and adolescents. They are most common in 50 and 60 year olds.3,6,9,10 Multiple aneurysms are found in 20 to 30% of patients with aneurysmal SAH.11–16 Factors associated with increased risk of formation of multiple aneurysms include hypertension, smoking, female sex, and age.13,14,17,18 The Unruptured Aneurysm The International Study of Unruptured Intracranial Aneurysms Reports Because of the widespread use and increasing sensitivity of imaging technology, a growing number of patients are diagnosed with unruptured, asymptomatic intracranial aneurysms. How these patients should be counseled is a hotly debated issue, prompted largely by the results of the International Study of Unruptured Intracranial Aneurysms (ISUIA).5,14 Patients harboring unruptured aneurysms have a potentially devastating condition, but the decision to treat the asymptomatic lesion requires a careful comparison of the risks of rupture to those associated with intervention. The ISUIA was a combined retrospective and prospective study, published in two parts, primarily designed to address the relationship of aneurysm size to risk of rupture.5,14 The retrospective data from the first report showed that aneurysm rupture risk depended on the size and location of the aneurysm and the presence or absence of a previously ruptured aneurysm elsewhere. The goal of the prospective arm of the study was to determine the natural history of unruptured aneurysms and the morbidity and mortality associated with treatment. Size and location were the most important variables in predicting annual incidence of SAH, although the incremental size categories were changed. Age was not a significant factor in the risk of rupture, and a history of SAH had no effect on rupture rates except in the smallest size category. Based on the findings from the retrospective study, the determined critical size of the “dangerous” unruptured aneurysm was set at 10 mm, unless there was a history of prior SAH from an aneurysm elsewhere, or the lesion arose from a posterior circulation or PComArt location. 19 With the publication of the prospective natural history data, the minimum size for treatment was lowered to 7 mm. These findings and treatment recommendations struck many as flawed, biased, and not representative of those commonly seen by practicing neurosurgeons. Nonetheless, these studies emphasize that the rupture risk from incidental aneurysms, while still undefined, is likely significantly lower than previously portrayed, particularly at certain locations. The studies also indicate that morbidity, mortality, and efficacy rates differ for the intervention and the practitioner. Therefore, treatment options for each individual patient must be carefully weighed and compared with the natural history of unruptured aneurysms left untreated. The Ruptured Aneurysm Subarachnoid Hemorrhage The incidence of aneurysmal SAH varies from 6 to 26 per 100,000 population per year; Finland and Japan report the highest values.20–30 Women outnumber men by a ratio of 1.6:1 in most large series,31,32 although men predominate before age 50.33 In the United States, the peak age for aneurysm rupture is between 40 and 60 years.6,31,32,34–36 The annual incidence rate increases from 3 per 100,000 population in 30 year olds to 30 per 100,000 in 60 year olds.21,24,26–28 For survivors of the acute hemorrhage, the untreated lesion carries a mortality rate during the first 2 weeks of 20 to 30%, with an additional morbidity rate of ~20%.32,35,36 Rebleeding is a major cause of this early morbidity and mortality; the risk of rebleeding during the first 2 weeks is ~20% and increases to 33% at 1 month and 50% at 6 months.32,36–39 The mortality rate for the untreated lesion is ~50% during the first year following rupture. The risk of rebleeding diminishes thereafter (estimated 3% annually); the mortality rate associated with second hemorrhage is 40 to 50%.7,37 Up to 25 to 35% of patients are found dead after the initial hemorrhage.6,7,18,32,40 Warning signs are reported in 15 to 60% of cases and may be attributed to minor hemorrhage (or “sentinel leak”), aneurysmal expansion, or ischemia.41–43 Half of these events occur within a week of the eventual SAH, and 90% within 6 weeks of SAH.41,44 The obvious consequence of missing the warning event is a missed opportunity to prevent a neurologically devastating hemorrhage. When considering the treatment options of a particular aneurysm, several general anatomical factors should be analyzed. Saccular aneurysms typically have a narrower “neck” and a broader, rounded fundus. A small, narrow-necked aneurysm is amenable to both surgical and endovascular obliteration. Such shapes are particularly favorable for endovascular treatment, because the narrow neck makes it easier to pack the aneurysm fundus with a dense coil mass without coil prolapse into the parent artery or associated branch vessel. When the aneurysm neck is broad compared with the maximal diameter of the aneurysm, however, endovascular intervention is less likely to be curative, as the broad neck makes dense packing of the aneurysm difficult and coil prolapse into the parent artery or associated branch vessel more likely. New stent and balloon-assist techniques increase the endovascular obliteration rate for these lesions, but such techniques are also more hazardous than coiling alone, and open surgical reconstruction becomes preferable in some instances. Most large or giant aneurysms (10 to 25 mm or >25 mm, respectively) are currently better treated surgically. The durability of endovascular techniques is poor, often due to progressive compaction of the coil mass within the fundus of an aneurysm caused by arterial pressure waves that enter through a relatively large arterial orifice at the neck of the lesion. Larger lesions with intraluminal thrombus are very prone to this phenomenon; when managed by endovascular methods, retreatment is commonly required. Anterior circulation aneurysms are generally associated with characteristic sets of very small perforating vessels, and recognition of the associated perforating arteries is critical to successful treatment. Surgical obliteration must spare these perforators to avoid a potentially devastating neurological injury. In certain locations (e.g., superior projecting anterior communicating artery aneurysms), the dissection and identification of these perforators are more difficult, and endovascular treatment may be preferred if other features of the aneurysm are also favorable to endovascular occlusion. Some aneurysms compress adjacent neural structures (e.g., optic or oculomotor nerves) or rupture to produce potential life-threatening increased intracranial pressure from an associated intraparenchymal hematoma. In these instances, endovascular techniques may obliterate the aneurysm, but mass effect is not eliminated; open surgical treatment is often preferable. The Unruptured Aneurysm In the ISUIA data, the 30-day morbidity and mortality associated with open surgical treatment of unruptured aneurysms (with or without a prior history of SAH) was 11% and 13.7%, respectively. Endovascular treatment showed morbidity and mortality at 7% and 9.3% for the same groups. The 1-year morbidity and mortality after open surgical treatment declined to 10.1% and 11.6%, and to 7.1% and 9.8% for endovascular treatment, respectively. Significant treatment risk factors associated with poor outcome were age >50, aneurysm size >12 mm, and posterior circulation location. The natural history and results of treatment, however, are far from homogenous, with variations present depending on location, aneurysm morphology, patient characteristics, and experience of the treatment team. The decision to treat an unruptured aneurysm as well as how to treat an individual lesion should be made by a physician who is aware of the treatment options and their risks, compared with the available data about the risk of rupture without treatment. Clearly, the ISUIA data confirms that age is a significant risk factor for surgery; any patient over 65 years of age with an unruptured aneurysm should be given strong considerations for endovascular treatment when treatment is considered. The Ruptured Aneurysm Physicians traditionally assign a clinical grade to patients presenting with ruptured saccular aneurysms, using the Hunt and Hess grading system that heavily emphasizes level of consciousness at time of presentation (Table 8-2). A major limitation, however, is that the scale was devised before the advent of computerized tomography (CT) scanning; therefore, it does not differentiate between altered states of consciousness due to systemic derangement, hydrocephalus, or hemorrhage extent.45–47 A clinical grade calculated after maximal cardiopulmonary resuscitation and intracranial pressure (ICP) control with a ventriculostomy is actually a better predictor of ultimate outcome. Table 8-2 Clinical Grading of Subarachnoid Hemorrhage* Grade Condition I Asymptomatic or with mild headache II Moderate or severe headache, nuchal rigidity III Confusion, drowsiness, or mold focal deficit (discounting third nerve palsy) IV Stupor or hemiparesis, early decerebrate rigidity V Deep coma, extensor posturing Aneurysmal SAH patients are generally divided into good (Hunt and Hess grades I, II, and III) and poor (Hunt and Hess grades IV and V) clinical grades.48 Following the results of the International Study on the Timing of Aneurysm Surgery (ISTAS), early surgery is now the standard of care for most patients.6,32,49 This standard has been reinforced by studies demonstrating that in good-grade patients (Hunt and Hess grades I to III), the combination of early surgery, calcium antagonists, and hypervolemic–hypertensive therapy can reduce overall management mortality to 10% or less, with good outcomes in 75% or more of those who survive.50–53 However, approximately 20 to 40% of patients with aneurysmal SAH fall in the poor grades; the association between poor outcome and poor clinical grade following SAH is clear.48,54,55 Data suggest that 35 to 50% of poorgrade patients have a reasonable neurological outcome with aggressive therapy, particularly if the determination of clinical grade is made before the insertion of a ventriculostomy.48,54–56 The initial clinical and radiographic findings are frequently inaccurate predictors of outcome; hence, aggressive treatment is warranted until the likelihood of irreversible brain injury is clarified. The brain of the high-grade SAH patient is often swollen and congested with bloody cerebrospinal fluid (CSF) throughout the subarachnoid space, and such a “tight” brain does not tolerate surgical manipulation or retraction well. Poor-grade patients, therefore, are generally best treated with ventriculostomy and endovascular intervention in most instances, unless a large, lifethreatening clot is contributing to the obtundation. Radiographic evidence of permanent injury incompatible with functional survival (e.g., a large hematoma in the dominant basal ganglia), sustained elevated ICP greater than 50 mm Hg, or poor or absent intracranial filling of large or critical vascular territories on angiography are contraindications to surgical or endovascular treatment of any type. Diagnostic Evaluation When SAH is suspected, evaluation should include a clinical history, general physical and neurological examination, routine laboratory studies (electrocardiography, electrolytes, complete blood count, coagulation tests), CT scan (lumbar puncture if the CT scan is negative), and some type of procedure demonstrating the details of the intracranial vessels (computed tomographic angiography [CTA], magnetic resonance angiography [MRA], or four-vessel cerebral angiography). A CT scan is the first diagnostic test to be ordered when bleeding is suspected, as hemorrhage can be detected in ~95% of patients with aneurysm rupture if the study is obtained within 24 hours.57,58 The distribution of blood suggests the aneurysm site in most cases and allows accurate predictions of the risk of subsequent vasospasm. A CT scan also clarifies associated conditions such as intracranial hematoma, edema, and hydrocephalus. It can also be extremely useful in clarifying the exact anatomical relation of the aneurysm to the skull base or the presence of thrombus or calcification within the aneurysm or parent vessel. A negative CT scan does not rule out SAH, and if the history is highly suggestive of an aneurysmal hemorrhage, lumbar puncture is mandatory. Evidence of hemorrhage in the CSF (xanthochromia) persists for 1 to 2 weeks, depending on the severity of the hemorrhage. High-quality four-vessel angiography remains unsurpassed for diagnosis and surgical planning. Preoperative angiographic studies determine several key features: (1) the aneurysm’s presence, location, size, shape, and relationship to parent and adjacent arteries; (2) the presence and distribution of vasospasm; (3) displacement of adjacent vessels suggesting mass effect from hematoma or partial thrombosis of an aneurysmal sac; and (4) the presence of other aneurysms or other vascular abnormalities. As many as 20 to 30% of patients have multiple aneurysms; therefore, a complete four-vessel study should be performed unless the patient’s condition dictates otherwise. Computerized tomographic angiography (CTA) is rapidly gaining ground as an adjunct or even an alternative to conventional arteriography. While potentially not as sensitive as conventional angiography for the detection of small aneurysms, this technology is noninvasive and often quicker to obtain, making it more valuable in the patient acutely declining from an intraparenchymal hematoma who needs urgent intervention. 59 For patients with unruptured aneurysms, CTA and MRA combined with CT and magnetic resonance imaging (MRI) are excellent screening tests, and transfemoral arteriography may not be required when study quality is excellent, thereby sparing exposure to the risks of arteriography. FIGURE 8-2 Algorithm for evaluation and management of anterior circulation aneurysms. Treatment Options: Preoperative Assessment, Management, and Timing of Surgical Intervention Unruptured asymptomatic anterior circulation aneurysms are treated electively, combining the preferences and schedules of both the patient and physician. Symptomatic lesions, however, are treated much more urgently, as the recent change in clinical status (i.e., recent oculomotor nerve dysfunction associated with an enlarging PComArt aneurysm) is often a harbinger of an upcoming hemorrhage. Our algorithm for the perioperative management of ruptured anterior circulation aneurysms is outlined in Figure 8-2. Initial management is focused on the conditions that could be acutely fatal or cause permanent injury, including hypoxia (from seizures, respiratory depression, and cardiovascular dysfunction) and increased intracranial pressure (from hydrocephalus and/or hematoma). Good-grade patients (Hunt and Hess grades I to III) harboring lesions judged to be best-treated surgically are treated within 24 hours after admission, unless other major clinical problems (i.e., myocardial dysfunction, pneumonia, etc.) or severe angiographic vasospasm are evident. In our opinion, all grade III or higher patients should have an immediate ventriculostomy, followed by intervention (either open surgical or endovascular) within 24 to 48 hours unless a life-threatening clot requires emergent evacuation. Great care is taken to prevent hypovolemia and hypotension in the perioperative period, especially during the interval of highest vasospasm incidence (4 to 10 days after SAH). General Operative Techniques: Single and Multimodal Surgical approach to anterior circulation aneurysms is best performed through a pterional craniotomy. The details of the general surgical technique have been described previously. What follow here are some recent observations regarding site-specific nuances that affect surgical approach and that may sway the decision process away from open surgical clipping and toward endovascular therapy. Site-Specific: Single and Multimodal Proximal (Paraclinoid) Internal Carotid Artery Aneurysms (Clinoidal and Ophthalmic Segments) Anatomy, Terminology, and Aneurysm Variants Ophthalmic segment aneurysms occur as three variants: ophthalmic artery, superior hypophyseal, and dorsal carotid wall (Fig. 8-3). Ophthalmic artery aneurysms originate just beyond the takeoff of the ophthalmic artery and point dorsomedially toward the lateral half of the optic nerve.60–63 Superior hypophyseal artery aneurysms arise from the inferomedial surface of the ophthalmic segment and have no relationship to the ophthalmic artery origin.61–63 One (parasellar) variant burrows inferiorly and medially toward and below the diaphragma sella, expanding into the “carotid cave.” When small, this variant is covered by parasellar dura, and its risk of SAH is extremely low. Once these lesions reach a size or projection sufficient to extend medially into the suprasellar space above the diaphragma sella (suprasellar variant), hemorrhage risks increase.61–63 The dorsal variant is the least common type and arises along the dorsal ICA surface well distal to the ophthalmic artery origin. Some appear to be pure hemodynamically induced saccular lesions, while others are blister-like and may represent dissections.60–63 Ophthalmic segment aneurysms are very common, perhaps the most common aneurysm found in women. Most are small and asymptomatic, and hemorrhage risks are lower than for aneurysms arising elsewhere. Symptomatic lesions present in roughly equal proportions between visual symptoms and SAH; those presenting with visual deficits are usually giant (≥ 25 mm). Clinoidal segment aneurysms are divided into the anterolateral and medial variants64 (Fig. 8-4). The former arises from the anterolateral surface of the clinoidal segment as it ascends between the carotid-oculomotor membrane (COM) and the dural ring (DR) medial to the anterior clinoid process (ACP). The hemodynamic vector on this ICA portion promotes a superomedial projection that can erode into or through the optic strut and ACP, compressing the ipsilateral optic nerve within the optic canal or secondarily reaching the subarachnoid space.61 The medial variant projects medially, below the diaphragma sella, into the pituitary fossa.61 Larger lesions can cause pituitary dysfunction or eventually expand into the subarachnoid space or sphenoid sinus. Careful interpretation of radiographic studies, including CT and MRI scans as well as cerebral arteriography, is required for an accurate differentiation between subtypes of clinoidal and ophthalmic segment aneurysms. The indications for intervention vary among paraclinoid aneurysm subtypes (e.g., small clinoidal segment lesions are not treated as aggressively as most ophthalmic segment aneurysms), as several variants have extremely low hemorrhage risks at that size. In addition, surgical technique differs between aneurysm variants (e.g., exposure of the cervical internal carotid artery, degree of clinoid removal), and each has specific problem areas of the exposure required for safe clip obliteration. A ruptured ophthalmic segment aneurysm usually produces hemorrhage within the chiasmatic and parasellar cisterns or, rarely, a focal clot within the orbitofrontale gyrus; because of the medial projection, bleeding is sometimes more prominent contralateral to the origin of the aneurysm.61,63 Intraluminal thrombosis or calcification must be clarified, as well as the bony anatomy of the anterior clinoid process (e.g., demonstrable erosion of the anterior clinoid process or optic strut suggests an anterolateral variant clinoidal segment aneurysm). An MRI scan can provide anatomical information about the aneurysms relation to the optic nerves or pituitary gland.65 Four-vessel transfemoral cerebral arteriography is very helpful in defining the anatomic characteristics of lesions in this region and in defining other aneurysms elsewhere in the circulation. Multiplicity is common; up to 50% of patients with a paraclinoid aneurysm will have at least one other aneurysm elsewhere, often in the contralateral paraclinoid region. Even when combined with three-dimensional reconstructions, however, the precise type of aneurysm can be difficult to differentiate (i.e., small medial variant clinoidal segment vs. parasellar variant superior hypophyseal lesions). The cervical carotid artery should be inspected for atherosclerotic disease that would make temporary clamping treacherous. The superficial temporal artery should be inspected for its possible use as a bypass conduit. Ophthalmic artery aneurysms arise from the dorsal surface of the internal carotid artery, above the posterior bend and just distal to the origin of the ophthalmic artery (Fig. 8-5). Superior hypophyseal artery aneurysms arise from the inferior or inferomedial surface of the carotid artery and project medially or posterior-medially toward and above the sella. Dorsal carotid wall aneurysms project superiorly, similar to ophthalmic artery aneurysms, but they originate from a portion of the carotid artery distinctly separate from the origin of the ophthalmic artery. Clinoidal segment aneurysms appear as a “double density” overlying the anterior ascending vertical segment of the ICA (Fig. 8-6). On antero-posterior (AP) projection, the anterolateral variant projects laterally toward or through the anterior clinoid process, whereas the medial variant projects off the medial ICA surface into the pituitary fossa below the diaphragma sella. Enlargement into the subarachnoid space is often accompanied by an angiographic “waist” that marks the point where the aneurysm traverses the dura to enter the subarachnoid space.66 FIGURE 8-3 Ophthalmic segment aneurysms: (A) Lateral view. (B) Dorsal view. (C) Anteroposterior (AP) view. Ophthalmic artery (OphArt) aneurysms (hatched area 1) arise from the dorsomedial internal carotid artery surface just distal to the takeoff of the OphArt. SupHypArt aneurysms (hatched area 2) arise from the medial or inferomedial ICA surface distal to the OphArt in close association with the medially projecting superior hypophyseal perforators. Dorsal variant aneurysms (hatched area 3) are uncommon, usually purely hemodynamic lesions that arise from the dorsal ICA surface well distal to the OphArt origin and well apart from the optic apparatus. ACP, anterior clinoid process; AN, aneurysm; CN VI, abducens nerve; COM, carotid-oculomotor membrane; III, oculomotor nerve, IV, trochlear nerve; ON, optic nerve; PcomArt, posterior communicating artery; Pit, pituitary gland; V1, first division of the trigeminal nerve; V2, second division of the trigeminal nerve.

 Decision-Making
Decision-Making
 Natural History and Conservative Management
Natural History and Conservative Management
 Treatment Risks, Indications, and Contraindications
Treatment Risks, Indications, and Contraindications
 Treatment Strategies
Treatment Strategies
 Treatment Techniques
Treatment Techniques
Anterior Circulation Aneurysms
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


