Appetite
SET POINT
In spite of great fluctuation in the quantity and frequency of eating, we all maintain remarkable precision between energy expenditure and energy intake. Social factors, emotions, and time of day, as well as taste, satiety, and personal habits, influence our eating patterns, yet we maintain a reasonably stable body weight month after month. This is referred to as energy homeostasis or simply a metabolic “set point.”
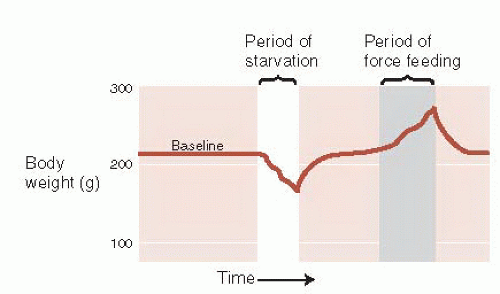
Evidence of a set point comes from a variety of sources. If a rat is deprived of food, then, when offered a normal diet, it will overeat for a short period and return its body weight to its preexisting level. Likewise, after being force-fed to increase body weight, it will limit its food intake to return to normal weight (Figure 13.1). The brain and body work in harmony to keep the body weight at a specific point.
The human corollary to this is the disturbingly low success rates that people have with most diets. In a review of long-term efficacy of dietary treatment interventions for obesity, Ayyad and Anderson found that only 15% of the patients fulfilled at least one of the criteria for success 5 years after the study. Unfortunately, most people who diet will slowly return to their preexisting weight within 1 year.
Several lines of research suggest that the set point is genetically controlled. Adoption studies provide a unique way to separate the effects of genetics from environment on body weight by comparing children with their biologic parents and with the parents from the house in which they were raised. In an analysis of Danish adoptees, Stunkard et al. found a strong relation between weight class of the adoptees and the body mass of the biologic parents. However, there was no correlation between the weight class of the adoptees and their adopted parents, suggesting that genetic makeup, not environment, is a major determining factor in one’s body weight.
Yet, obesity was a rare condition 50 years ago. Clearly, our genes have not evolved in half a century. There must be other explanations for the significant change in body weight that is occurring in first-world nations. The Pima Indians of Mexico and Arizona can provide some insight on this issue.
The Pima Indians separated into two tribes approximately 700 to 1,000 years ago—one tribe remained in Mexico and the other settled in what is now known as Arizona. In spite of their similar genetic makeup, they have remarkably different average body weights. The Arizona Indians have a high prevalence of obesity and non-insulin-dependent diabetes mellitus, whereas their Mexican relatives are not overweight and have little diabetes. The difference can be best explained by understanding the divergent lifestyles these two populations developed.
The Mexican Pima Indians have remained in the mountains, continuing a traditional rural lifestyle. They exert considerable physical energy working the farms and eat a diet high in starch and fiber. The Arizona Indians, on the other hand, were forced to move onto reservations and abandon their former way of life. There they lead more leisurely lives and eat a diet high in fat and sugar.
The obesity problem that the Pima Indians suffer from has been attributed to a “thrifty gene”—a gene that promotes saving and storing calories. Two thousand years ago, such a genetic predisposition would enhance the survival for those struggling with the fluctuations of prosperity and famine. However, with plentiful high caloric, highly palatable food, the “thrifty gene” promotes accumulation and storage beyond what is healthy. So the weight problem is genetic, but influenced by what is available in the environment.
The concept of a set point helps us understand the difficulties encountered by anyone trying to diet. One of the mechanisms the brain uses to return the body weight to its baseline set point became apparent in the Minnesota Starvation Experiment. This was an experiment conducted with conscientious objectors in Minnesota toward the end of the Second World War to help the military understand the condition of the starving civilians in Europe.
Participants were subjected to a semistarvation diet for 6 months with the goal of losing approximately 25% of their weight. From our perspective, one of the more interesting symptoms these men experienced during the experiment was obsessive thought about food. Not unlike the cravings discussed in the previous chapter, food became the principal topic of their thoughts, conversation, and daydreams. Reading cookbooks and collecting recipes became an intensely interesting pastime for many men. Eating, either mentioned in a book or shown in a movie, was a cue that put the men at heightened risk for breaking their diet. The urge to eat was so powerful that the researchers established a buddy system so that participants would not be tempted to cheat when away from the dorm. Clearly, their brains were focusing on what the body needed.
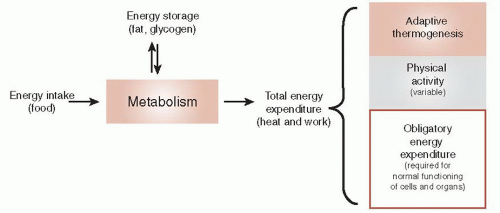
The brain can also adjust the energy expenditure as another way to maintain a stable body weight. Figure 13.2 shows a thermodynamic perspective of energy expenditure. Energy enters an organism as food and exits as heat or work. Energy is stored as fat or glycogen and mobilized when needed. Total energy expenditure can be subdivided into three components: obligatory energy expenditure, physical activity, and adaptive thermogenesis.
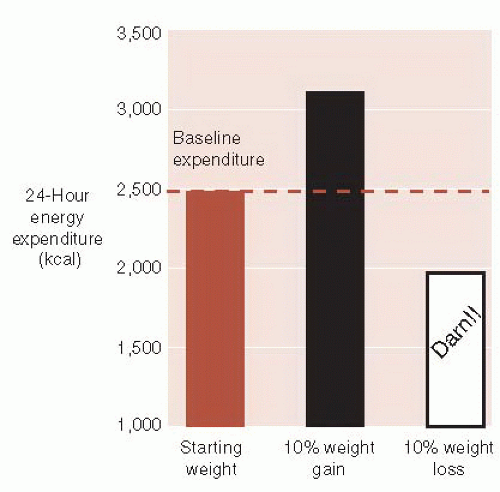
Adaptive thermogenesis is the other major obstacle the brain uses to thwart weight loss. Changes in sympathetic tone alter energy expenditure. For example, activation of the sympathetic nervous system results in catabolic breakdown of adipose tissue and produces energy. Figure 13.3 shows the changes in energy expenditure in healthy subjects after a 10% alteration in body weight. Not only does the brain increase adaptive thermogenesis when the body gains weight but also turns it down in response to weight loss—a frustration many dieters have experienced.
HOMEOSTATIC MECHANISMS
The key point is that despite large day-to-day fluctuations in food intake and energy expenditure, our weight remains within a relatively narrow range.
This is accomplished through feedback loops between the brain and the body. Brain lesion and stimulation studies in the 1940s identified the hypothalamus as a major center controlling food intake. Although the initial studies turned out to be overly simplified, the hypothalamus remains an important command center for weight maintenance.
This is accomplished through feedback loops between the brain and the body. Brain lesion and stimulation studies in the 1940s identified the hypothalamus as a major center controlling food intake. Although the initial studies turned out to be overly simplified, the hypothalamus remains an important command center for weight maintenance.
But what are the signals the brain receives and sends? Interrupting or enhancing these signals may provide opportunities for interventions to stem the obesity epidemic. The best way to understand the current conceptualization of the central nervous system (CNS) homeostatic mechanisms is to separate the short-term signals from the long-term ones.
Short-term Signals
The short-term signals tend to affect meal size rather than the overall energy storage. The signals comprise nutrients and gut hormones in the circulation as well as afferent signals sent up the vagus nerve. These signals to the brain result in the sensation of satiety but do not produce sustained alteration in body adiposity.
Nutrients
Glucose is the primary nutrient that mediates satiety. Hypoglycemia increases hunger sensations and stimulates eating. Glucose infusions will decrease the food intake. Other nutrients in the systemic circulation, such as fats and amino acids, play a real but limited role in signaling to the brain the effects of a recent meal. High levels of these nutrients tell the brain to stop eating, but the brakes are insufficient when the individual has been starving.
Mechanoreceptors
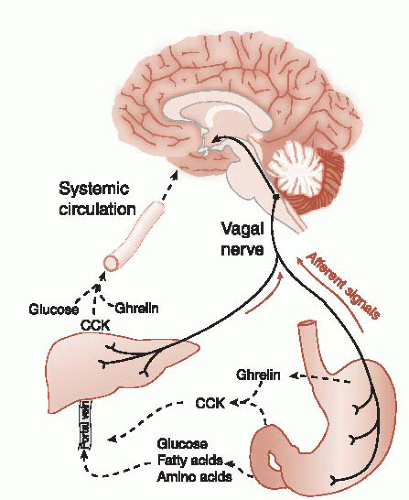
The physical presence of food in the stomach and upper small intestine activates mechanoreceptors. The stomach wall is innervated with stretch receptors that increase in activity in proportion to the volume in the stomach. We usually think of the vagus as a conduit from the brain to the gut, but as much as 80% of the neural traffic is flowing in the opposite direction (see Figure 2.9). The vagus nerve transmits signals about gastric distension to the hindbrain. This may be the reason some patients with vagus nerve stimulators report weight loss.
Gut Hormones
Numerous gut hormones are involved in food intake regulation. The most widely studied hormone is cholecystokinin (CCK). CCK is released from endocrine cells in the mucosal layer of the small intestine in response to fats and proteins. CCK inhibits further food intake through several mechanisms, such as stimulating the vagal nerve and inhibiting gastric emptying. Additionally, there are CCK receptors in the brain. The injection of CCK directly into the ventricles will inhibit eating. CCK appears to have central and peripheral mechanisms to put the brake on a meal.
Although CCK will limit food intake, its longterm administration does not induce significant weight loss. In studies with rats, the repeated administration of CCK resulted in smaller but more frequent meals. Thus, the overall energy balance was not altered. Figure 13.4 summarizes the short-term signals regulating food intake.
There are many other gut hormones that also inhibit eating, for example, glucagon-like peptide-1 (GLP-1) and peptide tyrosine-tyrosine. Ghrelin is of most interest as it is the only gut hormone that stimulates hunger. Produced in the stomach, fasting increases the levels of ghrelin, which then fall after a meal. Peripheral and central administration of ghrelin increases the food intake. In contrast to CCK, there is some evidence that ghrelin has longterm effects on weight and may be a potential culprit in obesity. Some studies suggest that reduced ghrelin production is one of the reasons gastric bypass surgery is so effective (see Figure 13.10).
The Joy of Eating
Eating is more than just sustenance; it is one of the great pleasures of life. The perception of pleasure that we get from some foods is most likely an adaptation that enhanced the survival of our ancestors
during lean times. However, with the abundance of inexpensive highly palatable refined foods, genes that favored sweet foods cause us to overeat.
during lean times. However, with the abundance of inexpensive highly palatable refined foods, genes that favored sweet foods cause us to overeat.
Certain foods increase dopamine at the nucleus accumbens (see Figure 12.6). Additionally, the endogenous opioids appear to be more active during a good meal. Other evidence suggests that eating is a highly valued pleasure that can resemble an addiction. For example, obese individuals have reduced D2 receptors in the striatum and activation of the orbitofrontal cortex when craving food. Clearly, dopamine and the endogenous opioids are signals that influence energy consumption.
Long-term Signals
Long-term signals tell the brain about the overall energy storage, not just the caloric content of the recent meal. In the 1950s, it was suggested that adipose tissue releases a hormone that signals the hypothalamus about the current state of energy storage. Termed an adiposity signal, the elusive hormone must have the following three traits:
Circulate in the blood in proportion to the amount of stored fat
Cross the blood-brain barrier and stimulate specific receptors in the brain
Produce changes in caloric intake and energy expenditure when levels of the hormone fluctuate
DISORDERS
YOUR GRANDFATHER’S DIET
A remarkable study from an isolated community in Sweden has shown that a man’s risk of death from cardiovascular disease and diabetes is affected by his grandfather’s diet during his grandfather’s slow growth period—ages 9 to 12 for boys. Using records of harvest success or failure during the 19th century, the researchers found increased risk of heart disease and diabetes if the grandfather lived through bountiful harvests during his slow growth period. Alternatively, grandfathers who grew up during times of famine sired grandchildren who lived longer. The mechanism, derived in part from rodent studies, is believed to be transgenerational epigenetic inheritance (see Figure 6.12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree