Fig. 26.1
Overnight polysomnographic (PSG) recording in a patient with upper airway obstructive sleep apnea syndrome (OSAS) showing recurrent episodes of mixed apneas (initial control followed by obstructive events) during stage 2 NREM (N2) sleep. Top 4 channels show electroencephalograms (international nomenclature). (A1, left ear; A2, right ear; Loc, left electro-oculogram; ROC, right electro-oculogram; CHIN, submental electromyogram (EMG); EKG, electrocardiogram; LT TA, left tibialis anterior EMG; LT GA left gastrocnemius EMG; RT TA, right tibialus anterior EMG; RT GA, right gastrocnemius EMG; ORON, oronasal airflow; PFLO, nasal pressure transducer recording airflow; THOR, chest respiratory effort; ABDM abdominal respiratory effort; SaO2, Oxygen saturation (%) by finger oximetry; SNOR, snoring sound recording. l

Fig. 26.2
PSG recording from a 70-year-old woman showing the classic crescendo–decrescendo pattern of Cheyne–Stokes breathing (CSB) during stage 2 NREM sleep. The presence of CSB throughout most of the NREM sleep (with marked decrement or absence during REM sleep) in this patient with a history of hypertension and excessive daytime sleepiness suggests occult left ventricular failure. The PSG montage is similar to that in Fig. 26.1
Overnight PSG findings in patients with narcolepsy include short sleep latency, excessive disruption of sleep with frequent arousal, reduced total sleep time, excessive body movements, reduced slow-wave sleep, and sleep-onset REM (seen in 40–50 % of patients). Some narcoleptic patients may have associated sleep apnea, particularly central apnea. In approximately 9–59 % of patients, PLMS has been noted, and in up to 36 % of narcoleptic patients RBD has been described.
The characteristic PSG findings in RBD consist of absence of muscle atonia and presence of increased phasic electromyographic (EMG) activities in the upper and lower limbs during REM sleep. It is important to record EMGs from both upper and lower limbs because, in some patients with RBD, excessive EMG activities are present in the upper limbs but not in the lower limbs.
In RLS, PSG findings document sleep disturbance and PLMS (see Fig. 26.3), which is found in at least 80 % of patients. Diagnosis of periodic limb movement disorder is based on the PLMS index (number of periodic limb movements per hour of sleep) of 15 and over plus symptoms of sleep disturbance. A high PLMS index with arousal is more significant than an index without arousal.
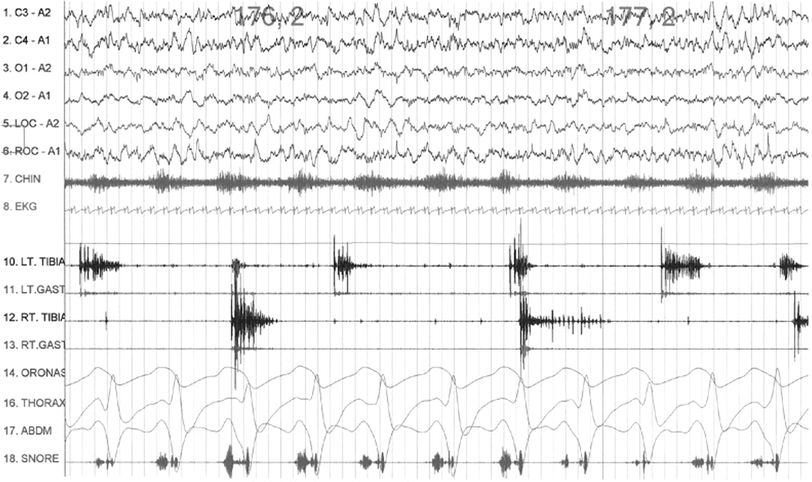

Fig. 26.3
Polysomnographic (PSG) recording showing periodic limb movements in sleep (PLMS) characterized by dystonic and dystonicmyoclonic electromyographic (EMG) bursts in left (LT) and right (RT) tibialis (TIBIA) and gastrocnemius (GAST) muscles during stage 2 (N2) non-rapid eye movement (NREM) sleep in an adult patient with restless legs syndrome (RLS). Top 4 channels show electroencephalograms (EEG) using international nomenclature. (A1, left ear; A2, right ear; LOC, left electroculogram; ROC right electro-oculogram; CHIN, submental EMG; EKG electrocardiogram; ORONAS, oronasal airflow; THORAX, chest respiratory effort; ABDM, abdominal respiratory effort (Reproduced with permission from Chokroverty [64])
Pitfalls of PSG
PSG is the single most important laboratory test for assessment of sleep disorders, particularly in patients presenting with EDS and those suspected of nocturnal seizures, parasomnias, or other abnormal motor activities. However, PSG has considerable limitations. There is no standardized uniform protocol used consistently in all sleep laboratories, making the comparison of the data from one laboratory to another somewhat misleading. The most severe limitations are that an overnight in-laboratory PSG is labor-intensive, time-consuming, and expensive. A single-night PSG may miss the diagnosis of mild OSAS, PLMS, parasomnias, and nocturnal seizures. PSG data and the patient’s clinical findings may not be concordant. Standard PSG study cannot diagnose upper airway resistance syndrome definitively. PSG cannot determine the etiology of apnea–hypopnea syndrome. PSG is not helpful in the diagnosis of insomnia, the most common sleep disorder in the general population. PSG is not helpful in the diagnosis or treatment of circadian rhythm sleep disorders. PSG data may be confounded by first-night effects (e.g., increased wakefulness and stage 1 NREM sleep, and decreased slow-wave and REM sleep). Standard PSG does not measure arterial partial pressure of CO2 and thus may miss hypoventilation, which is an early abnormality (particularly REM hypoventilation) in neuromuscular disorders. Standard PSG does not adequately measure cardiac function (one channel of electrocardiography [ECG] is inadequate), which may affect the prognosis of OSAS. Furthermore, cardiorespiratory sleep studies, which do not include EEG, may produce false-negative findings in mild-to-moderate OSAS patients. Standard PSG does not include autonomic monitoring, which may be important for assessment of autonomic activation as well as for assessing autonomic changes that are intense during sleep.
Video-Polysomnographic Study
A video-PSG study is important for documenting abnormal movements and behavior during sleep at night in patients with parasomnias, including RBD, nocturnal seizures, and other unusual movements occurring during sleep [62]. Parasomnias are generally diagnosed on the basis of a clinical history, but sometimes a video-PSG study is required to document the condition. For suspected nocturnal epilepsy, a video-PSG study using additional electrodes to include multiple-channel EEG and multiple montages covering both parasagittal and temporal regions bilaterally is required for optimal detection of epileptiform activities. Ideally, if sleep epilepsy is suspected, video-PSG recordings should be interpreted using EEG analysis at the standard EEG speed of 30 mm/sec to identify epileptiform discharges. Multiple EMG channels to record from additional muscles (e.g., forearm flexor and extensor muscles, masseter and other muscles) for patients with suspected RBD and bruxism are recommended.
Video-PSG may help characterize the movements, differentiate one jerk from another, identify a specific entity, and most important, differentiate abnormal motor activities from nocturnal seizures. Video-PSG may aid in the diagnosis of other coexisting sleep disorders (e.g., OSAS, RBD, narcolepsy). Video-PSG does help us classify abnormal motor activities during sleep into several identifiable entities (e.g., motor parasomnias, nocturnal seizures, involuntary diurnal movements persisting during sleep, PLMS, excessive fragmentary myoclonus seen in a variety of sleep disorders, dissociative disorders, nocturnal jerks, and body movements seen in patients with OSAS). Many parasomnias may be mistaken for nocturnal seizures (e.g., confusional arousals, sleepwalking, sleep terror, sleep talking, bruxism, rhythmic movement disorder, RBD, nightmares, and dissociative disorders). RBD and nightmares occur during REM sleep. These conditions can be diagnosed and differentiated from one another based on characteristic clinical features combined with EEG and video-PSG findings. Box 26.16 lists indications for video-PSG.
Box 26.16 Indications for Video-PSG
Unusual and complex arousal disorders
Complex behaviors suspicious of RBD but not absolutely certain based on the history
Behavior and motor events at night suggesting possible nocturnal seizure disorder
EDS in patients with epilepsy, to determine whether excessive sleepiness is due to repeated nocturnal seizures, an undesirable side effect of antiepileptic medications, or an associative sleep disorder (e.g., sleep apnea)
Suspected psychogenic dissociative disorder
Sleep-related movement disorders (e.g., rhythmic movement disorders, bruxism), which may be mistaken for nocturnal seizures
Involuntary diurnal movement disorder persisting during sleep
Coexisting secondary sleep disorder (e.g., narcolepsy and RBD; OSAS and sleepwalking; narcolepsy and sleep apnea)
For medicolegal purpose when the patient presents with violent behavior during sleep; video-PSG studies are mandatory to evaluate suspicions for correct diagnosis of parasomnias or seizure disorders.
Multiple Sleep Latency Test
The MSLT is an important test to effectively document EDS (see Chap. 22). Narcolepsy is the single most important indication for performing MSLT. The presence of two or more sleep-onset REM periods (SOREMP) from four or five nap studies (a SOREMP in the preceding overnight PSG study may substitute for one MSLT SOREMP) and sleep-onset latency of less than 8 min strongly suggest a diagnosis of narcolepsy [30] (Fig. 26.4). Abnormalities of REM sleep regulatory mechanisms (e.g., OSAS, insufficient sleep syndrome, use of REM-suppressant medications) or circadian rhythm sleep disturbance may also lead to REM sleep abnormalities during an MSLT.
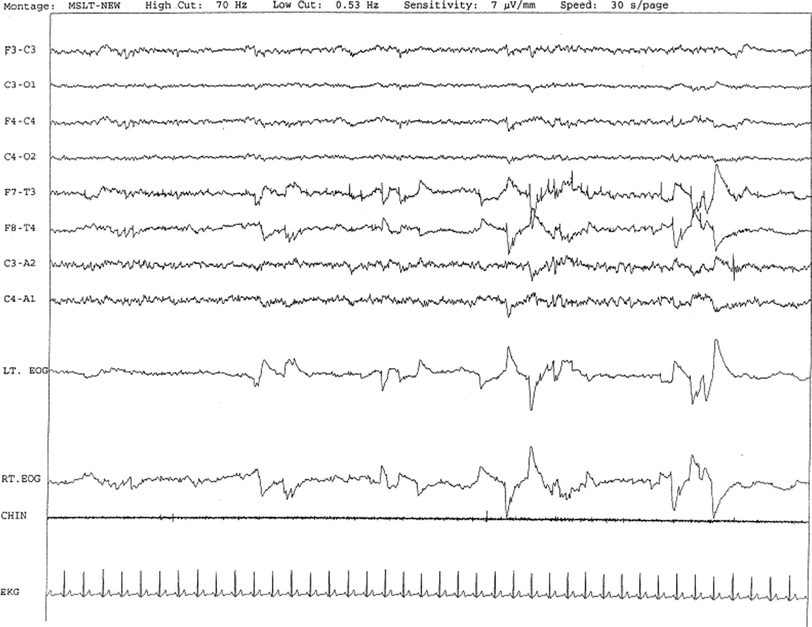

Fig. 26.4
A case of narcolepsy in a 60-year-old woman with new onset of intermittent episodes of sudden transient bilateral leg weakness, excessive daytime sleepiness, and intermittent periods of transient confusion. A daytime EEG was normal. Overnight PSG was significant for sleep architecture changes with an immediate sleep-onset latency, presence of only 1 REM cycle, with a decreased REM sleep percentage (7 %), an increased arousal index of 23 without associated apnea or periodic limb movements, and excessive fragmentary myoclonus in NREM and REM sleep. The MSLT showed a mean sleep latency of 1.6 min, consistent with pathologic sleepiness, and the presence of 2 (of 4) sleep-onset REM naps suggestive of REM sleep dysregulation as seen in narcolepsy. A 30-sec epoch from the MSLT showing the presence of sleep-onset REM occurring 7 min after sleep onset. Prominent REMs are seen in the EOG channels and anterior temporal EEG electrodes. Eye movements, characteristic of REM sleep, are noted as described. CHIN, electromyography of chin; EEG, top eight channels; EKG, electrocardiography; Lt. and Rt. EOG, left and right electro-oculograms. (Reproduced with permission from Chokroverty [64])
Maintenance of Wakefulness Test
The maintenance of wakefulness test (MWT) is a variant of the MSLT (see Chap. 23), measuring a subject’s ability to stay awake. It also consists of four to five trials of remaining awake occurring every 2 h. Each trial is terminated if no sleep occurs after 40 min or immediately after the first 3 consecutive epochs of stage 1 NREM sleep or the first epoch of any other stage of sleep [61, 62]. If the mean sleep latency is less than 8 min, it is then considered an abnormal test. The MWT is less sensitive than the MSLT as a diagnostic test for narcolepsy but is more sensitive in assessing the effect of treatment (e.g., CPAP titration in OSAS and stimulant therapy in narcolepsy).
Standard Electroencephalographic Study
An EEG is necessary to investigate suspected epilepsy (see Chap. 44).
Ambulatory Electroencephalography or Polysomnography
Ambulatory EEG or PSG is sometimes useful for patients with suspected sleep epilepsy, for understanding circadian variation and for studying circadian rhythm sleep disorders. However, technical problems associated with unattended recording are serious limitations.
Actigraphy
This is another laboratory test for assessing sleep disorders that use an actigraph (also known as an actometer) worn on the wrist or ankle to record acceleration or deceleration of body movements (Fig. 26.5), which indirectly indicates the state of sleep or wakefulness. The actigraph can be worn for days or weeks, and this test complements a sleep log or diary in diagnosing circadian rhythm sleep disorders (Fig. 26.6) and in assessing patients with insomnia (Fig. 26.7), including paradoxical insomnia or sleep state misperception; inadequate sleep hygiene; and prolonged daytime sleepiness. Actigraphy is useful to document rest–activity patterns over days and weeks when a sleep log is not able to provide such data. Advantages of actigraphy over PSG include the following: easy accessibility; inexpensive recording over extended periods of days, weeks, or months; recording of 24-h activities at all sites (home, work, laboratory); usefulness in uncooperative and demented patients when laboratory PSG study is not possible; ability to conduct longitudinal studies during therapeutic intervention (e.g., cognitive behavioral therapy or pharmacologic treatment) in patients with insomnia; usefulness in paradoxical insomnia; and ability to document delayed or advanced sleep-phase state or circadian rhythm sleep disorder, free-running type. Although not adequately standardized, actigraphy may have a role in patients with RLS and PLMS.

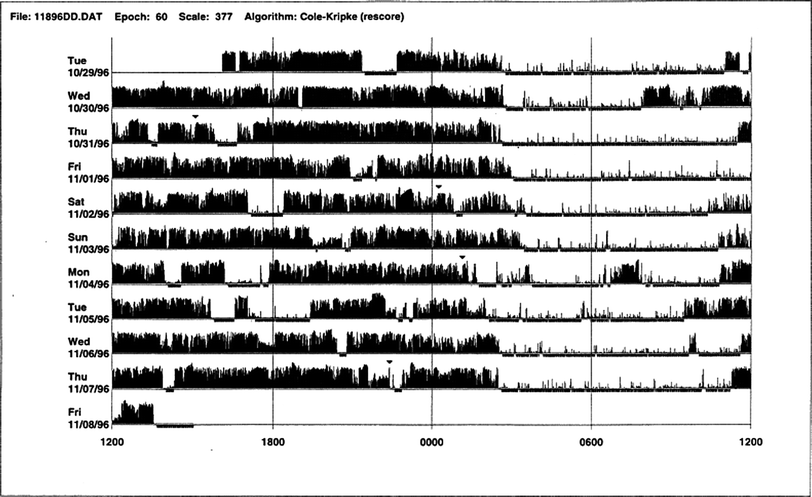


Fig. 26.5
Normal sleep–wake schedule. This wrist actigraphic recording from a 50-year-old healthy woman without sleep complaints shows a fairly regular sleep–wake schedule. She goes to bed between 10:30 and 11:00 p.m. and wakes up around 6:30 a.m. except on the fourth and eighth day. Physiologic body shifts and movements during sleep are indicated by a few black bars in the white areas. The waking period is indicated by black bars

Fig. 26.6
Primary delayed sleep-phase syndrome. This wrist actigraphic recording is taken from a 29-year-old man with a lifelong history of delayed sleep onset and delayed wake-up time. The actigram shows his typical sleep period from 3:00 a.m. to 4:00 a.m. to 9:00 a.m. to noon (white areas). If he has to wake up early in the morning, he feels exhausted and sleepy all day. He feels fine if he is allowed to follow his own schedule. Melatonin at night did not help him. Morning bright light therapy was suggested but the patient declined. (Reproduced with permission from Chokroverty [64])

Fig. 26.7




Actigraphy in insomnia (sleep state misperception). A 59-year-old man complaining of insomnia since the age of 12 years was diagnosed to have an Axis 2 personality disorder (dependent personality) according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, as well as panic attacks, and is being treated with benzodiazepines (diazepam 3 mg, flurazepam 30 mg) and zolpidem 10 mg. He denies any symptoms of restless legs syndrome (RLS), excessive daytime sleepiness, or daytime sleep attacks. Subjective sleep duration is 3–4 h per night. In the past, he had used numerous drugs for sleep amelioration, but no clear and stable subjective improvement was noted. Actigraphic monitoring (during drug dosage reduction: diazepam 2 mg, flurazepam 15 mg, and no zolpidem) shows a clear misperception of sleep duration and quality. The recording shows normal nocturnal motor activity and sleep efficiency and duration; note sleep period during the afternoon. He complained of sleeping not more than 3 h each night. PSG on the third night revealed the following: TST, 387 min; SE, 73.5; WASO, 122 min; number of awakenings, 17; SWS %, 1.3; PLMS index, 1.9. (PLMS, periodic limb movements in sleep; PSG, polysomnography; SE, sleep efficiency; SWS, slow-wave sleep; TST, total sleep time; WASO, wake after sleep onset.) (Reproduced with permission from Chokroverty [64])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








