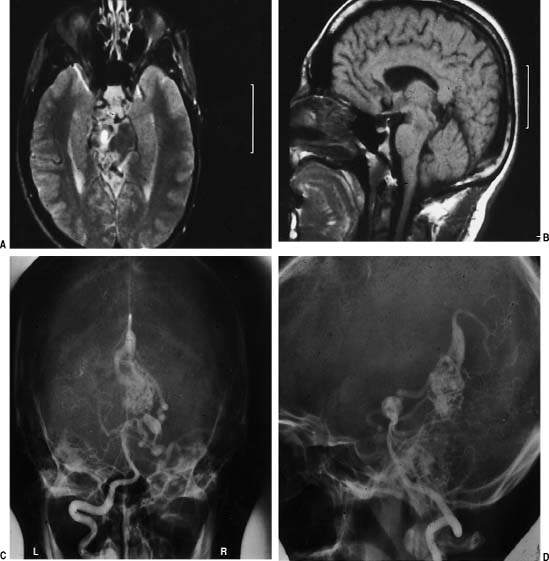
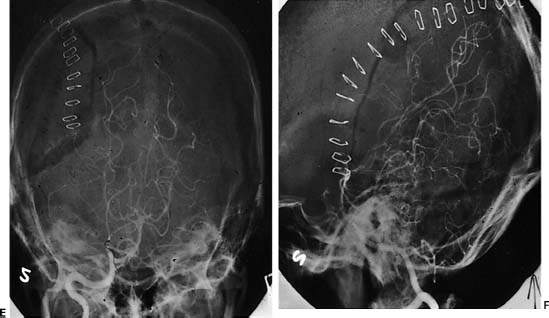
Surgical Treatment of Cerebral Arteriovenous Malformations Objectives: Upon completion of this chapter, the reader should be able to explain how to select patients for treatment or conservative management and how to develop appropriate treatment strategies that combine embolization, microsurgery, and radiosurgery. Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians. Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity. The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp * The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons. Arteriovenous malformations (AVMs) are vascular abnormalities leading to a fistulous connection of arteries and veins without a normal intervening capillary bed. In the cerebral hemispheres, they frequently occur as cone-shaped lesions with the apex of the cone reaching toward the ventricles. Nearly all AVMs are thought to be congenital. Supratentorial location is the most common (90%). The most common presentation of an AVM is intracerebral hemorrhage (ICH). After ICH, seizure is the second most common presentation. Other presentations of AVMs include headache and focal neurological deficits that may be related to steal phenomena or other alteration in perfusion in the tissue adjacent to the AVM, such as venous hypertension from arterialization of normal draining veins. In managing unruptured AVMs, it is important to understand the natural history of these vascular malformations. The decision for no treatment or for a single or multi-modality treatment paradigm also involves being familiar with the outcomes and risks of each treatment modality—microvascular resection, endovascular embolization, and stereotactic radiosurgery. Finally, the patient-related factors, such as age, general medical condition, neurological condition, and occupation, must also be taken into consideration before reaching a conclusion. The treatment of AVMs is highly individualized. There is no universal algorithm or protocol to be followed when dealing with these unique problems. The currently used treatments for AVMs include (1) microsurgical resection only, (2) preoperative endovascular embolization followed by microsurgical resection, (3) stereotactic radiosurgery only, (4) preprocedural endovascular embolization followed by radiosurgical treatment, (5) endovascular embolization only, and (6) observation only. The ultimate goal for all of these modalities is cure for the patient; however, the only way to achieve cure is with complete obliteration of the AVM. Microsurgery is the gold standard for resection of small superficial AVMs against which other methods of treatment must be measured. There is certainly a well-established role for adjunctive endovascular embolization of some AVMs. Clearly, there are specific situations, such as small deep AVMs in eloquent brain structures, where microsurgery should not be used as the primary treatment modality; stereotactic radiosurgery and occasionally embolization (when there is reasonable expectation of complete obliteration by embolization) are the preferred treatment options in these cases. We also make a case for observation in patients with large AVMs in or near critical areas of the brain that are not ideal for surgical resection or radiosurgery. Here, the pursuit of treatment may actually be more harmful to the patient than the natural history of the AVM. Several series have evaluated the natural history of AVMs with regard to the risk of hemorrhage. In a series of 168 patients without a history of previous hemorrhage, 18% of patients had subsequent hemorrhage over a mean follow-up of 8.2 years.1 Annualized hemorrhage rate was 2.2%. In a study reported by Graf et al, hemorrhage risk at 1 year was 2%, at 5 years was 14%, and at 10 years was 31%.2 A retrospective study of 217 patients with AVMs followed for an average of 10.4 years yielded an annual hemorrhage rate of 3.4%.3 An important study by Ondra et al outlined the natural history of AVMs among 160 patients who presented with symptomatic AVMs, which were followed for a mean follow-up of 23.7 years.4 This study included 160 patients who presented mainly with hemorrhage; they were followed conservatively for an average of 24.7 years. The mean age at presentation was 33 years. The rehemorrhage rate was 4% per year, with an average of 7.7 years for the next hemorrhage to occur. The yearly morbidity rate was 1.7%, and the mortality rate was 1%. This study demonstrated the high morbidity and mortality associated with AVMs regardless of initial mode of presentation including hemorrhage, headache, or seizure. The only prospective study of the natural history of AVMs resulted in an annual hemorrhage rate of 2.2%. However, follow-up of this group of 139 patients was short at an average of only 1 year.5 Table 12-1 summarizes the previously published studies on the natural history of AVMs. Several angioarchitectural factors influence the risk of hemorrhage for AVMs. Diagnostic Evaluation A computed tomography (CT) scan may be used as an initial screening tool for patients presenting with neurological sequelae related to unruptured or ruptured AVMs. This study can be used quickly to determine location of the lesion, acute hemorrhage, hydrocephalus, or areas of encephalomalacia from previous surgery or rupture. A nonenhanced CT may show irregular hyperdense areas frequently associated with calcifications in unruptured AVMs and acute hemorrhage on plain CT scan with ruptured AVMs. With the addition of intravenous contrast material, a CT scan shows the nidus and feeding vessels or dilated draining veins. Magnetic resonance imaging (MRI) is superior to a CT scan in delineating details of the macro-architecture of the AVM except in the case of acute hemorrhage. These architectural features include exact anatomic relationships of the nidus, feeding arteries, and draining veins as well as topographic relationships between AVM and adjacent brain6 (Fig. 12-1A–F). MRI is sensitive in revealing subacute hemorrhage.7 The AVM appears as a sponge-like structure, with patchy signal loss, or flow voids, associated with feeding arteries or draining veins on T1-weighted sequences. MRI and angiography in combination provide complementary information that facilitates understanding the three-dimensional structure of the nidus, feeding arteries, and draining veins. Magnetic resonance angiography (MRA) currently cannot replace conventional cerebral angiography. In the case of acute hemorrhage, the hematoma obscures all details of the AVM, making MRA virtually useless. This calls for direct use of cerebral angiography if the characteristics of the hematoma strongly suggest AVM as an etiology. Complete cerebral angiography with multiple projections is a mandatory step in the preoperative evaluation of a patient with an AVM. Cerebral angiography can localize the nidus, the feeding arteries, and the draining veins. Angiography may assess the flow dynamics within the nidus of the AVM. The search for associated aneurysms is part of the preoperative evaluation. External carotid injections to determine the presence of an external supply are necessary in cases of large-convexity AVMs. It is important that the angiogram be performed close to the time of surgery, as AVMs can change in size and configuration over time. Vessels that were not seen secondary to compression from a hemorrhage may appear on a follow-up angiogram weeks later. Many techniques are available for studying the functionality of cortical structures surrounding the AVM. These include the use of positron emission tomography (PET), functional MRI (fMRI), magnetoencephalography, and direct provocative testing of cortical function. Judicious utilization of these techniques will enhance the safety of AVM therapy. Such information may allow the surgeon to tailor treatment modalities to increase the margin of safety during treatment and to decrease periprocedural flow-related hemorrhagic or ischemic complications.8 FIGURE 12-1 The role of correct localization of the arteriovenous malformation is shown. On this axial T2-weighted magnetic resonance image (MRI) (A), it is not clear whether this lesion is in the midbrain. Sagittal T1-weighted MRI (B) shows that this pial tectal arteriovenous malformation is not located in the midbrain parenchyma. Because of this anatomical location we decided to operate on this patient. The anteroposterior and lateral angiograms (C, D) show that this arteriovenous malformation is fed mainly by superior cerebellar artery branches. The postoperative AP (E) and lateral (F) angiogram shows complete obliteration. Size of Arteriovenous Malformations In a series of 168 patients followed after presentation without a prior hemorrhage, the size of the AVM was not found to be predictive of future hemorrhage, utilizing a multivariate statistical analysis.9 However, other studies have found AVMs of small size to be at higher risk of hemorrhage. Spetzler et al compared the feeding artery pressures in small and large AVMs.10 They found higher feeding artery pressures in the small AVMs and suggested that small AVMs bleed more often than large ones. However, the question of whether this is the case is still controversial. Draining Veins Deep drainage has been thought to be an important risk factor for hemorrhage from an AVM. Nataf et al reported a strong correlation between frequency of hemorrhages and presence of deep drainage in AVMs.11 Arteriovenous malformations with a single draining vein were found to have a higher risk in some studies.12,13 This can be explained by the fact that impaired drainage through a single vein leads to a high risk of hemodynamic overload and eventual rupture. Impairment in venous drainage caused by stenosis or kinking may also increase the risk of bleeding.13 Arteriovenous Malformations and Aneurysms Prevalence of the association of AVMs with aneurysms varies from 2.7 to 22.7%. This association seems to be correlated with a higher risk of hemorrhage. Brown et al studied 91 patients with unruptured AVMs. Among these, 16 patients had 26 saccular intracranial aneurysms.14 The researchers found the risk of ICH in patients with coexisting AVM and aneurysm to be 7% at 1 year compared with 3% among those with AVM alone. At 5 years, the risk persisted at 7% per year, while it decreased to 1.7% per year in those with an AVM unassociated with aneurysms. Ninety-six percent of 26 aneurysms were located on an AVM arterial feeder. The significance of intranidal arterial or venous aneurysms, which are quite common in large complex AVMs, is unknown, although it has been suggested that this finding may be associated with an increased risk of hemorrhage.15 Indications for Surgical Resection There are several clear indications for microsurgical resection of AVMs. Arteriovenous malformations with Spetzler–Martin grades I to III on the convexity should generally be resected. The Spetzler–Martin grading system takes into account three factors that greatly affect the surgical resectability of the AVM: size (<3 cm, 1 point; 3–6 cm, 2 points; >6 cm, 3 points), location (non-eloquent cortex, 0 points; eloquent cortex, 1 point), and venous drainage (superficial only, 0 points; deep, 1 point).16 Table 12-2 summarizes the methodology for the Spetzler–Martin grading system. Table 12-2 Methodology of Spetzler–Martin Grading of Arteriovenous Malformations Feature Characteristic Points Size >3 cm 1 Venous Drainage Superficial 0 Location Noneloquent cortex 0 However, these are not the only factors that the neurosurgeon must take into account when considering treatment options for an AVM. There are other AVM-related factors, such as the presence of AVM-associated aneurysms and venous restrictive disease, and patient-related factors, such as age, general health, and occupation/avocation. In addition, the specific experience and expertise of the surgeon and the center where treatment is to take place must be taken into account. Each AVM and patient must be considered individually. There is no magic formula to dictate to a physician and surgeon how to proceed in managing a patient. For example, deep venous drainage may actually be an advantage intraoperatively, as the draining veins are hidden away from the surgeon until the last moments of AVM removal. Patients with AVMs that present with major hemorrhage, progressive neurological deterioration, inadequately controlled seizures, intractable headache, or venous restrictive disease should be strongly considered for surgical resection.17 There is a caveat to operating on ruptured AVMs leading to large intracerebral hemorrhages and significant neurological deficits. These patients should be operated on in a delayed fashion to allow for maximal recovery of neurological function and accurate determination of preoperative neurological status. It is clear that the risk of rebleeding is relatively low (~6% within the first 6 months) during this waiting period. In comparison, the benefits of waiting are great for the patient and surgeon. At the plateau of neurological recovery, the surgeon will then have a true sense of the patient’s preoperative neurological condition. What we want to try to avoid is the loss of potential for future neurological recovery by operating early on a patient with intracranial hemorrhage from a ruptured AVM. For example, the patient may be hemiplegic after a hemorrhage from the basal ganglia; significant recovery may occur spontaneously, and it is unfair to the patient to remove the AVM early under the presumption that “He is hemiplegic, I can’t make him worse.” The surgeon, under this rationale, may turn a reversible hemiplegia into a permanent deficit. Arteriovenous malformation treatment should be strongly considered in patients with intractable seizures or, in rare cases, intractable headaches, as these symptoms are likely a hindrance to daily living activities. The chance of relieving the symptoms of these patients and giving them a normal life back may outweigh the risks of surgery. Patients with venous restrictive disease may present another strong argument for surgical excision. With the occlusion of venous outflow from the nidus of the AVM, the intranidal hemodynamics begin to change. Acutely, pressure begins to rise in different compartments of the AVM, and chronically, new, fragile venous draining pathways are recruited. These changes are likely to increase the risk of AVM hemorrhage. Cerebellar and pial brainstem AVMs should also be given strong consideration for surgical resection to prevent the higher risk of bleeding as compared with supratentorial AVMs.17 A case may also be made for treatment of some basal ganglia and thalamic AVMs, as they carry an annual bleed rate of 11.4%, considerably higher than the average bleeding rate when AVMs in all locations are considered. In addition, morbidity and mortality with each bleed in these locations reach 7.1% and 42.9%, respectively (again, in contrast to the overall mortality rate of AVM hemorrhage of 10%).18,19 The above comments about indications are only to suggest that in these cases stronger consideration should be given to treatment as opposed to observation. However, we want to emphasize that all AVMs, whether they have bled or not, causing symptoms or not, should be considered for treatment. The basis for this statement is the well-known fact that, after the first few months of a hemorrhage, the risk of hemorrhage is the same for AVMs that have bled as for those that have not.4,20 As always, the ultimate recommendation should rest on the balance between the presumed risk of treatment and the risk of future hemorrhage or progressive disability, taking into account the multiple factors discussed and, very specifically, the likely number of years at risk if the AVM is left untreated, which obviously is directly related to the age and general health of the patient.20 As emphasized above, decision-making in determining the best management pathway for patients harboring AVMs must include consideration of the patient’s age, general health and clinical condition, occupation and lifestyle, location and size of AVM, surgeon’s experience, and ethical considerations.20,21 The patient’s age is most important in determining the cumulative risk of AVM rupture during the remainder of the patient’s life expectancy. Assuming an annual hemorrhage rate of 2 to 4% and an average life expectancy of 70 years, the cumulative risk (in percentage) of AVM rupture may be estimated by the following formula: 105 minus the patient’s age in years.22,23 Hence, one may justify a more aggressive approach for surgical treatment in younger patients, as their cumulative risk of hemorrhage is so high. In addition, neurological deficit caused at a young age is generally better tolerated and has a greater chance of recovery. The general health of the patient is important, as a patient with severe comorbid conditions may preclude surgery as a reasonable treatment option. The clinical presentation and neurological condition of a patient will often dictate timing of surgery; for example, the patient may need emergent evacuation of a hematoma caused by a ruptured AVM, or it may be best to wait until the patient has improved to a neurological plateau when AVM resection can be approached electively. The occupation and lifestyle of a patient are important considerations as the neurosurgeon begins to weigh the risks and benefits of treatment of an AVM in a critical area of the brain. For example, a patient who is a pilot and is dependent on his or her sharp visual acuity presenting with an occipital AVM may feel differently about surgical resection with a >50% chance of causing a postoperative hemianopsia than a patient with an AVM in the same location who is a housewife. For obvious reasons, AVM microstructure and surgeon’s experience are important factors in determining a treatment strategy. Ethical considerations relate to surgeon’s experience and come into play at a point where the surgeon determines if an AVM is operable or inoperable. The latter decision should preferably be made by an experienced cerebrovascular neurosurgeon at a referral center who specializes in AVM surgery. The surgeon should be familiar with the literature as well as his or her own personal experience and should be able to explain to the patient all treatment options with their associated risks and benefits. Importantly, the surgeon should inform the patient clearly and unambiguously of what, in his or her opinion, is the best treatment option, which in certain cases may be no treatment at all. In general, AVM surgery is elective. As discussed above, we recommend operating on ruptured AVMs that lead to intracranial hemorrhage and significant neurological deficits in a delayed fashion. We have seen many “good” results reported after excision of large AVMs of the thalamus and basal ganglia operated on early after a hemorrhage that rendered the patient hemiplegic. The thinking is that the hemorrhage has already destroyed critical areas of the brain that lead to devastating neurological deficits, and therefore, surgery cannot do further harm to the patient. “Good results” in these instances frequently mean that the patient’s neurological condition was the same as before surgery. However, it is possible that the patient’s preoperative condition would have changed for the better with time to recover from the ictus. Frequently, the hemorrhage does not destroy functional parts of the brain; instead, the mass from the hemorrhage splays apart gray and white matter, producing a deficit from pressure rather than destruction of critical brain. As the hematoma begins to resolve, these areas of the brain may recover to variable degrees. After a reasonable delay to allow such potential recovery to occur, the surgeon will be in a better position to judge whether, given the degree of the recovery, it may not be preferable to treat the patient with an alternative treatment modality (i.e., radiosurgery) or to recommend conservative therapy.24 Outcomes Microsurgical resection of Spetzler–Martin grades I, II, and III AVMs by experienced surgeons carries high cure rates and low complication rates with immediate elimination of risk of hemorrhage.25,26 Angiographic cure rate with microsurgery ranges from 94 to 100%. Microsurgery can achieve 100% angiographic obliteration for unruptured convexity AVMs <3 cm with superficial venous drainage.17 The combined surgical morbidity and mortality for AVMs grade I, II, and III is reported to be less than 10% in several large series.18,27–35 In a series of 110 patients harboring grades I to III AVMs taken to the operating room for microsurgical resection, 99% had angiographically confirmed obliteration of the AVM. Two patients (1.8%) required reoperation for residual AVM. Some authors have recommended immediate reoperation for residual malformations following surgical resection.36 The risk of neurological deterioration in the immediate postop period was 10.9% and declined to 2.7% by 6 months after surgery.18 In another series of small AVMs, 67 patients underwent microsurgical resection with a surgical outcome of 1.5% morbidity and 0% mortality.33 Pikus et al reported a series of 19 patients with small AVMs, grades I to III, yielding a 0% rate of morbidity and mortality.34 In our series of 311 patients who underwent microsurgical resection alone before 1993 for AVM, grades I to III patients demonstrated 89.9% good outcome, 9.5% significant disability, and 0.5% death.37 Grades IV and V patients demonstrated 60.7% good outcome, 37.5% significant disability, and 1.8% death. In a follow-up study of 153 consecutive patients with AVMs of all grades with a mean follow-up period of 3.8 years, we looked at the immediate morbidity and mortality rate and compared it to the late morbidity and mortality rate.28 The overall immediate postoperative of serious morbidity was 24.2%; the serious morbidity at follow-up was 7.8%. The mortality rate at follow-up was 1.3%. There was no history of intracranial hemorrhage in any patient during the followup period. At follow-up, 97.8% of patients with grades I to III AVMs were in good or excellent condition, 1.1% experienced a poor outcome, and 1.1% died. In the group of patients with grade IV and V AVMs, 79.0% had good outcome, 17.7% had poor outcome, and 3.2% died. Table 12-318,26–35,38 and Table 12-426–32,35,39–41 summarize the microsurgical outcomes from some of the larger published case series for Spetzler–Martin grades I to III and grades IV and V AVMs, respectively. Completely obliterated AVMs lead to the best outcome in terms of seizure control.42 After surgical excision, 81% of patients with a history of seizures were seizure free, whereas seizure-free outcome after radiosurgery and embolization was at 43% and 50%, respectively.43 Heros et al reported a seizure-free survival in patients with AVM experiencing preoperative seizures of 43.6%.28 Table 12-3 Postoperative Outcomes for Grades I to III Arteriovenous Malformations Author (Year) No. of Patients Morbidity and Mortality Rates Pik et al (2000)18 110 10.9% Early morbidity; 2.7% late morbidity Sisti et al (1993)33 67 (small AVMs) 1.5% Combined morbidity and mortality Pikus et al (1998)34 19 0% Nussbaum et al (1995)27 199 9.5% Early morbidity; 0.5% early mortality Heros et al (1990)28 91 1.1% Late morbidity; 1.1% late mortality Tokunaga et al (2000)29 8 0% For grades I and II; 75% early morbidity, 50% late morbidity, and 0% mortality in grade III Irie et al (2000)30 27 0% Hashimoto et al (2000)38 2 0% Hongo et al (2000)31 20 4% Mortality Russell et al (2002)35 35 8.6% Morbidity; 0% mortality Hartmann et al (2000)26 95 5.3% Morbidity; 0% mortality Hamilton and Spetzler (1994)32 71 0%

 Natural History
Natural History
 Surgical Resection
Surgical Resection
3–6 cm
>6 cm
2
3
Deep
1
Eloquent cortex
1
Arteriovenous Malformations
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


