Fig. 12.1
Non-contrast axial CT of brain showing a large intracerebral hemorrhage at time of presentation
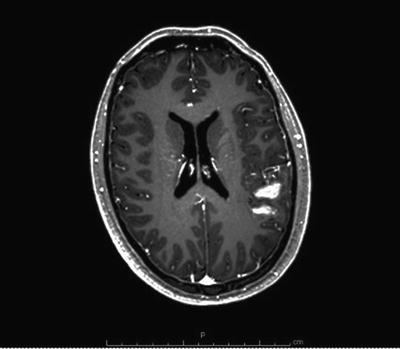
Fig. 12.2
Axial T1 MRI of the brain with gadolinium demonstrating a ruptured left frontoparietal AVM with associated hematoma
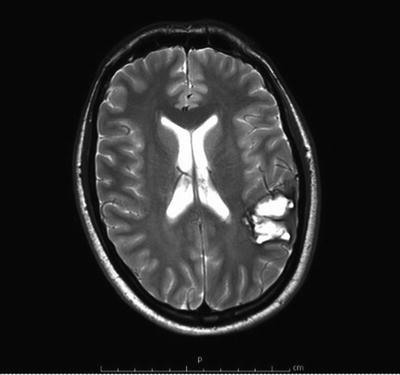
Fig. 12.3
Axial T2 MRI of the brain demonstrating a ruptured AVM with associated hematoma. Small vascular flow voids can be seen representing feeding vessels
Noninvasive vascular imaging such as CT angiography (CTA) and MR angiography (MRA) is also a widely used diagnostic testing for evaluation of AVMs. They are both more sensitive and specific than plain CT and MRI in visualizing AVM’s angioarchitecture. They remain however inferior to DSA in their ability to demonstrate the temporal flow relationship of the lesion to its surrounding vasculature. They can also miss low-flow small AVMs, which can only be confirmed with DSA [27, 39].
The flow dynamics of an AVM can be significantly altered by an acute hematoma. This may cause the size of the AVM to be underestimated or for the lesion to be missed entirely. Repeating the imaging after 6–8 weeks (after the hematoma resolves) may improve visualization [16, 43].
Noninvasive imaging techniques are inferior to conventional DSA in their ability to accurately characterize the hemodynamic behavior of an AVM including the exact location and size of its nidus, the number and flow rates of its various arterial feeders, and the location and characteristics of its draining veins relative to the normal vasculature (Fig. 12.4). All of these characteristics have huge therapeutic and prognostic implications, and their precise knowledge is crucial prior to any planned treatment. Moreover, DSA is superior in its ability to identify associated vascular anomalies such as extranidal and intranidal aneurysms, intranidal arteriovenous fistulas, and any associated vascular occlusive disease that may alter the treatment plan. DSA can also be used diagnostically in the preoperative planning of AVM treatment to test eloquence and map for potential posttreatment neurological deficits. This is done using provocative or superselective Wada testing (see Chap. 9) by locally delivering agents such as amobarbital and propofol among others intra-arterially into the AVM vasculature resulting in transient arrest of brain function in the region of local infusion. This is of particular importance in lesions lying in close proximity to language centers in the dominant hemisphere [44, 45]. Although invasive in nature, modern DSA has been shown to be extremely safe with a very low risk of complications and long-term sequelae [46].
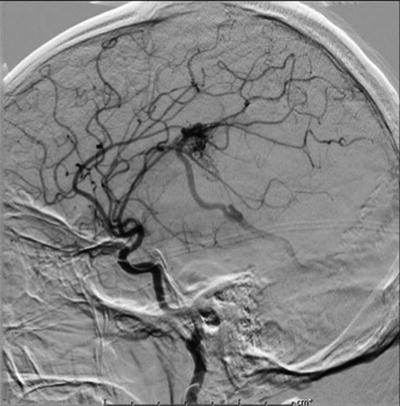

Fig. 12.4
A conventional DSA showing left frontoparietal AVM nidus supplied by feeders from the middle and anterior cerebral arteries. A single draining vein can also be seen flowing inferiorly and posteriorly
Therapeutic Decision Making
Multiple variables must be considered when choosing the best course of treatment, including: Spetzler–Martin grade (accounting for size, venous drainage, and eloquence), lenticulostriate supply, compactness of the nidus, the history of previous hemorrhage, and the patient’s clinical condition. Available options for treatment include microsurgery, radiosurgery, and endovascular therapy, as well as conservative management.
Classification
Multiple means of grading AVMs have been proposed with focus on using these methods to help guide therapeutic decision making. The most widely used system is the Spetzler–Martin scale (Table 12.1). This grading system was originally designed for risk stratification regarding surgical resection and is based on AVM size (<3 cm nidus, 3–6 cm, >6 cm), pattern of venous drainage (deep versus superficial), and eloquence of surrounding brain tissue (including sensorimotor, language, and visual cortex, hypothalamus, thalamus, internal capsule, brain stem, cerebellar peduncles, and deep cerebellar nuclei). AVMs are graded on a scale of I–V. Higher grades indicate a higher degree of surgical difficulty, with some AVMs classified as grade VI or inoperable [47].
Table 12.1
Spetzler–Martin grading system for AVMs
Characteristic | Points |
|---|---|
Size of nidus | |
<3 cm | 1 |
3–6 cm | 2 |
>6 cm | 3 |
Venous drainage pattern | |
Superficial only | 0 |
Deep | 1 |
Eloquence of adjacent brain | |
Non-eloquent | 0 |
Eloquent | 1 |
Evaluation of this grading system has been correlated with patient outcomes [1, 48–54]. A study by Hamilton et al. determined the permanent major neurological morbidity rates for grades I through III were 0 %, increasing to 21.9 % in patients with grade IV and 16.7 % in patients with grade V AVMs [48]. Comparable results were noted in a paper by Spears et al., with early disability (within 7 days) as follows: grade I, 2.1 %; II, 9.4 %; III, 17.3 %; and IV, 39.1 % (no grade V patients). Permanent disability was seen in 2.1 % of grade I patients, 5.7 % in grade II, 1.9 % in grade III, and 21.7 % in grade IV. Statistical analysis did not reveal a difference between outcomes in grades I–III in long-term outcomes [54]. Significant differences between lower grade AVMs were seen in a study by Hartmann et al., in which the study showed any long-term deficits (both mild and disabling) postoperatively in 8 % of grade I patients, 36 % in grade II, 32 % in grade III, and 65 % in grade IV [49]. Similarly, Heros et al. found morbidity in 1.9 % in grade I, 6.5 % in grade II, 23 % in grade III, 32 % in grade IV, and 69 % in grade V [50]. The overall trend does point to correlation with Spetzler–Martin grading; however, variations in the literature between each group have lead to proposed modifications in the system, ranging from expanding the classification to identify differences within groups [55] to simplifying the system into low-risk, moderate-risk, and high-risk categories to aid in capturing larger groups for statistical analysis as well as making application of the system easier [56, 57]. However, none of these modifications are currently widely used.
Microsurgery
Due to low risk of associated morbidity and mortality, surgery is generally recommended in lower grade AVMs. High-grade AVMs are less likely to be treated with surgical intervention due to perceived surgical risk. However, with advancements in surgical techniques and equipment, imaging and neuronavigation, and implementation of multimodality treatment, surgery in high-grade AVMs is becoming safer and more successful [58]. The primary of goal of surgery is the prevention of hemorrhage. Secondary goals are alleviation of seizures and preoperative neurological deficits; however, the efficacy of surgery with these indications is less clear since both can be complications of surgery as well [1, 58].
Surgery for AVMs is typically performed in an elective manner. While there are some reports of acute resection of AVMs after hemorrhage [1, 59, 60], it has been shown that allowing resolution of surrounding edema and removing the AVM in a planned, controlled fashion with a good understanding of its angioarchitecture are more likely to produce favorable outcomes. An ideal compromise can usually be accomplished with the presence of liquefied hematoma surrounding the AVM and absence of significant brain edema. The time between initial rupture and resection of the AVM usually increases in proportion to the size of the hematoma at time of rupture.
Exceptions may have to be made in cases of large hematomas causing significant mass effect and midline shift. An urgent craniotomy or craniectomy may be necessary. The appropriate approach has to be individualized in those cases, and the primary goal of the operation consists of maximal decompression. It is recommended not to resect the nidus in these cases unless a craniectomy and duraplasty are not sufficient [1, 59, 60].
Approach and positioning of the patient are dependent upon the location of the AVM, and skull-based approaches may be required for the best exposure. Surgery is greatly facilitated when it is possible to access the main arterial feeders early in the procedure and disconnect them prior to mobilizing the nidus (Fig. 12.5). Deep-seated AVMs are best approached with the assistance of frameless stereotactic navigation, which aids in planning optimal trajectory and size of craniotomy and confirms margins during resection. Its use has also shown decreased operative times and blood loss [61].
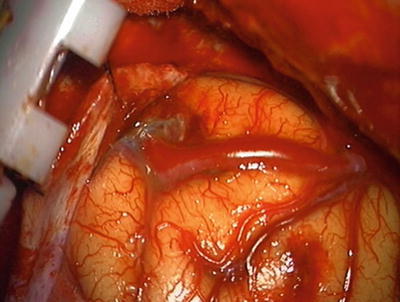

Fig. 12.5
Intraoperative photograph showing large superficial feeding artery
The craniotomy should be designed to achieve identification of superficial feeding vessels. Correlation with angiography is essential to help distinguish arteries from arterialized draining veins. Careful dissection of sulci, fissures, and subarachnoid cisterns should be performed to secure the more proximal portions of feeding vessels. These vessels should then be followed towards the nidus, where they are coagulated and divided, or clips can be applied (Fig. 12.6). Care should be taken to identify en-passage vessels, which supply normal brain tissue distal to the AVM. Small feeding branches to the AVM from these vessels should be identified and taken with the main artery preserved [1, 58].
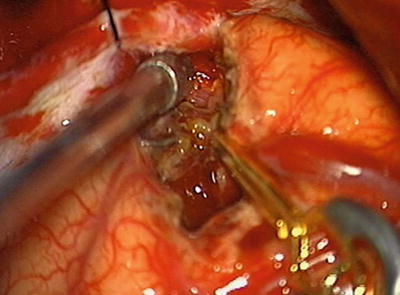

Fig. 12.6
Intraoperative photograph. Initial dissection of nidus with temporary clip placed on feeding vessel
Once the feeding vessels have been controlled, a circumferential dissection of the nidus is performed. The nidus should be separated from the underlying brain by taking advantage of the rim of gliosis that surrounds the nidus (Fig. 12.7). Initially the nidus is still under high pressure, so direct coagulation of the nidus can result in hemorrhage and should be avoided. Therefore, it is recommended to avoid coagulation of the nidus until sufficient numbers of feeding vessels have been disconnected and the nidus decreases in size and turgor. Deep perforators and feeding vessels can be a source of hemorrhage, and use of mini clips can be helpful to control those vessels and prevent them from retracting in the surrounding brain after incomplete anticoagulation. After the nidus has been disconnected from its inflow, it will appear deflated, and the venous drainage will have become darker. At this time, disconnection from the venous drainage system is indicated, and the nidus can be removed en bloc [1, 58].
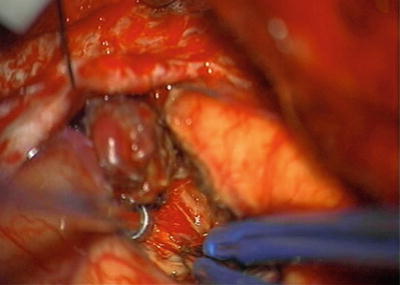

Fig. 12.7
Intraoperative photograph. Deep dissection of AVM with nidus retracted superiorly
Meticulous hemostasis is critical to the microsurgical resection of AVMs. Coagulating high-flow vessels is more difficult and requires longer application of cautery. After removal of the nidus, the cavity should be inspected for any potential sources of bleeding. Increasing the patient’s systolic blood pressure by 15–20 mmHg can assist in identifying points of breakthrough [1, 58].
Intraoperative confirmation of complete resection is desirable and can be achieved by either conventional digital subtraction angiography or intraoperative near-infrared indocyanine green (ICG) angiography. Use of conventional angiography requires placement of an arterial catheter and use of fluoroscopy and a radiolucent head holder, whereas ICG angiography requires intravenous administration of ICG and a microscope with integrated function. ICG angiography may have limited capacity to identify deep vessels or nidus hidden by surrounding parenchyma, but both have capability of identifying residual nidus and differentiating normal from residual AVM vessels and are useful tools for assessment of total resection [1, 58, 62].
Endovascular Treatment
Introduction
The endovascular approach to treatment of AVMs consists of percutaneous transarterial delivery of therapeutic embolic agents that are introduced locally into the AVM nidus or its feeding and draining vessels with the ultimate goal being hemodynamic shutdown of the AVM. The site of entry into the body is usually the femoral artery or in some instances the brachial or radial arteries. Endovascular embolization of AVM has been shown to be an invaluable tool in the pre- and post-microsurgical and radiosurgical management of AVMs and in certain cases can serve as the definitive curative treatment [63–65].
Embolization Strategy
Endovascular treatment of AVMs assumes one of three roles: adjunctive, curative, or palliative. The extent of embolization desired or achieved depends on a number of factors including: (1) lesion characteristics including size, accessibility, and the number and size of feeding vessels, (2) experience of the operating interventionalist, (3) available technology in terms of access systems and embolic agents, and in some instances (4) a therapeutic decision is sometimes taken to only partially obliterate the AVM if it is felt that complete obliteration carries more risk of morbidity or mortality.
Endovascular embolization is typically performed in multiple stages spanning weeks or even months. This approach reduces the risk of intracerebral hemorrhage as a complication that may result from treatment-related alteration in cerebral flow dynamics within the AVM and the surrounding normal parenchyma in the immediate vicinity. A mechanism known as normal perfusion pressure breakthrough explains this risk in which a sudden occlusion of a major AVM feeder leads to diversion of blood to adjacent parenchymal tissue that has been hypoperfused prior to treatment with maximally dilated normal vessels that in turn fail to autoregulate the sudden increase in diverted flow, leading to dangerous hyperperfusion and probable hemorrhage [66, 67].
Adjunctive embolization
Endovascular therapy is often utilized as part of a multimodality treatment approach to AVMs that also includes microsurgery and radiosurgery. This approach enables more successful treatment of deeply seated and large AVMs and has been shown to improve patient outcome [68–70]. The purpose of adjunctive embolization is to supplement to other modalities through reduction of the AVM nidus by shutting down some of its feeders. This can facilitate surgical excision of accessible lesions, help in preparation for radiosurgery of lesions that are initially too large to respond to radiation, and also be used to treat associated vascular lesions such as aneurysms [71–73]. As an adjunct to microsurgical resection, endovascular embolization has proven very helpful in cases of AVMs with a large nidus, deep-feeding vessels, and high-flow shunts (Figs. 12.8 and 12.9). This approach has allowed for safe treatment of AVMs with higher Spetzler–Martin grades as compared to surgical resection alone while at the same time shortening operative time and minimizing blood loss intraoperatively (Fig. 12.10) [63, 74, 75].
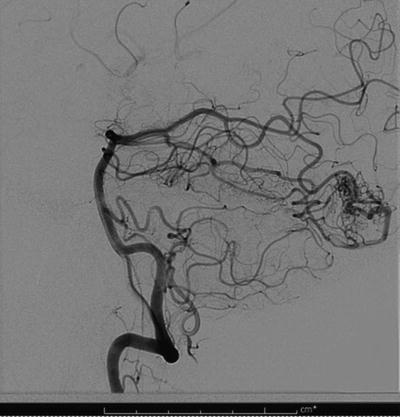
Fig. 12.8
A pre-embolization conventional angiogram showing occipital AVM with feeders from the posterior cerebral artery
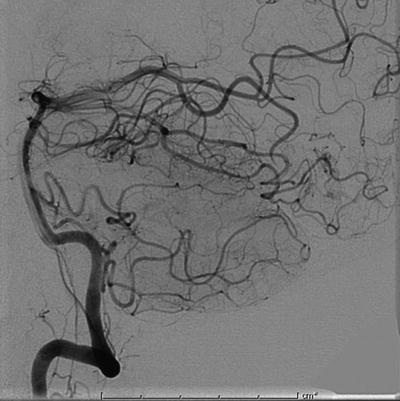
Fig. 12.9
Post-embolization conventional angiogram of the same patient demonstrating near-complete shutdown of the AVM nidus
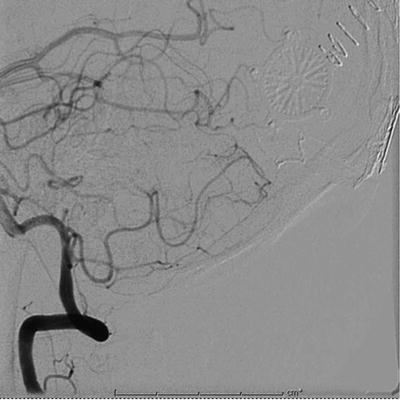
Fig. 12.10
Postsurgical resection angiogram of embolized occipital AVM
Endovascular embolization is one of the highest risk neurointerventional procedures and should not be offered without careful consideration. Some architecturally simple and small AVMs (Spetzler–Martin grades I and II) can be safely removed using surgical excision alone. On the other hand, embolization should be strongly considered in AVMs with a Spetzler–Martin grade of III, IV, and sometimes V and, as mentioned above, generally any AVMs with deep feeders that are hard to access through microsurgery [76]. As an adjunctive treatment, the degree of nidal occlusion does not always need to be 100 %. The work by Vinuela et al. suggests that while endovascular embolization is most useful to the surgeon when the AVM nidus has been occluded by at least 75 %, lesser degrees of occlusion were also helpful if they removed deep inaccessible feeders [77].
For AVMs that are large and deeply seated in eloquent cortex, multimodal treatment consists of endovascular embolization and radiosurgery. The goal of endovascular embolization in these cases is to reduce the size of the AVM in addition to treating associated vascular lesions that are not responsive to radiation, such as intra- and extranidal aneurysms and fistulas. Radiosurgery generally becomes more likely to achieve a cure as the size of the AVM nidus decreases. There is data to suggest that radiosurgical cure is more likely when the AVM nidus volume is reduced to less than 10 ml (diameter < 3 cm). Gobin et al. showed that embolization was most helpful as adjunct to radiosurgery in treatment of AVMs with a nidus size of 4–6 cm in diameter [78–81]. In some cases, endovascular embolization is used post-radiosurgery, in AVMs that fail to obliterate after radiosurgery [72, 81].
Curative
The concept of embolization as a curative modality is somewhat controversial. However, there is an increasing belief among interventionalists that complete angiographic obliteration leads to elimination of hemorrhage risk. Achieving this goal is challenging. The main reason for this is the difficulty in superselectively catheterizing and obliterating all of the small feeders that most AVMs have. In case series of AVMs destined for multimodal treatment with endovascular embolization as the initial therapy, only 10–20 % of these lesions were declared cured with embolization alone with no further treatment modality required [63, 79, 82]. However, and with the continuing evolution of endovascular equipment, technique, and tools for lesion accessibility and obliteration, data from more recent series demonstrated higher cure rates of 27–49 % [64, 83]. When specific criteria were used to select AVMs to undergo primarily curative endovascular embolization as opposed to its use as an adjunctive treatment, even higher cure rates were reported. These criteria included AVMs with a single nidus, with few prominent feeders, and with more fistulous rather than nidal arteriovenous shunting. Cure rates with endovascular embolization alone approaching 75 % were reported in such selected subcohorts [65, 82]. Wikhom et al. also suggest that the cure rate depends heavily on the volume of the nidus, with those smaller than 4 ml having over 70 % chance of cure as opposed to a 15 % cure rate for those larger than 4 ml [84].
Palliative treatment
In certain AVMs that are surgically inoperable or cannot be obliterated with multimodal treatment, palliative embolization may be offered to reduce the risk of recurrent hemorrhage posed by perinidal aneurysms or to alleviate neurological symptoms caused by local mass effect or steal phenomenon [85, 86]. Whether palliative treatment of AVMs that are asymptomatic improves the natural history of these lesions is controversial with strong data suggesting that it does not alter the natural history of these lesions [27, 86] and with some studies suggesting it may actually increase the risk of intracerebral hemorrhage [84, 87].
Tools Review: Embolic Agents (See Chap. 1)
Several embolic agents have been developed over the years to treat AVMs. Some of these are now almost obsolete due primarily to poor nidal penetration, higher recurrence rates, and an increased overall complication rate. Examples of such agents are silk sutures and polyvinyl alcohol particles (PVA) [88, 89]. The success of any embolic treatment lies mainly in the embolic agent’s ability to penetrate and durability. Proximal feeding vessel embolization without penetration into the nidus typically results in nidal recurrence via a phenomenon known as nidal recruitment in which the AVM nidus, over time, recruits new arterial feeders [90, 91].
This section will focus on the two most widely used, Food and Drug Administration (FDA) approved liquid embolic agents. These are N-butyl cyanoacrylate (n-BCA) (TRUFILL, Codman Neurovascular, Raynham, MA) and ethylene vinyl alcohol copolymer (Onyx, Covidien, Irvine, CA). Other embolic materials such as platinum coils are sometimes used in treatment of AVMs or associated aneurysms, but these are discussed elsewhere in this book.
N-butyl cyanoacrylate (n-BCA)
Approved by the FDA in 2000 for treatment of brain AVMs, n-BCA is marketed in the USA under the name TRUFILL® n-BCA Liquid Embolic System (Codman Neurovascular, Raynham, MA). It is also commonly referred to in the medical literature as “glue.”
Chemically, n-BCA is a liquid adhesive monomer that is clear and free flowing in its pure form. Upon contact with body fluids and tissues including blood, the monomer undergoes a rapid polymerization reaction via an anionic mechanism transforming it into a solid state that forms a hard cast inside the lumen of the containing structure or vessel.
The monomer is carefully injected under fluoroscopic guidance via superselective catheterization of the target vessel or nidus. The catheter is placed as close as possible to the nidus of the AVM in order to avoid hardening inside the feeding vessel prior to reaching and penetrating the nidus [92, 93].
Prior to its delivery, n-BCA is usually mixed in various ratios with an ethiodized oil compound to retard the polymerization reaction and to allow the injected mixture to travel some distance and achieve better nidal penetration before polymerization sets it. Once injected, the operator should be ready to retract the delivery microcatheter within seconds to prevent hardening of the mixture around the catheter tip and trapping the catheter tip within the artery, which can lead to retention of a catheter fragment upon attempted retrieval [67]. In addition to its role as an occlusive agent, it is also shown that n-BCA induces an inflammatory reaction in situ, promoting fibrotic remodeling and involution over time, thus aiding in the obliteration process [94].
EVOH (Onyx)
Onyx® LES is made of ethylene vinyl alcohol copolymer (EVOH) (Covidien, Irvine, CA). It is a liquid nonadhesive copolymer that received its FDA approval in 2005 for endovascular embolic treatment of AVMs. It solidifies inside the vessels from the outside inward, creating a semisolid shell. This process is analogous to the hardening of lava and led to its trade name, Onyx.
Onyx was mainly developed to address one main shortcoming of n-BCA: its rapid polymerization in contact with tissue. This property is not optimal for many users due to the perceived risk of trapping the delivery catheter within the embolic mass. The Onyx solidification process occurs over minutes to hours in a cohesive rather than adhesive manner. This allows more time and control for the operator treating the AVM while at the same time promoting more complete nidal penetration. Once it solidifies, the end product is a spongy cast within the injected lumen.
Onyx is delivered into the target vessel dissolved in dimethyl sulfoxide (DMSO). DMSO allows the copolymer to travel some distance once injected before it precipitates out of the solvent and begins the solidification process. The distance it travels depends on the final viscosity of the mixture (EVOH plus DMSO). Onyx is supplied in two different concentrations producing two different viscosities. Onyx 18 is composed of 6 % EVOH and 94 % DMSO producing a viscosity of 18 centipoises, and Onyx 34 is composed of 8 % EVOH and 92 % DMSO and has a viscosity of 34 centipoises. Onyx 34 therefore is more viscous, making it useful in the treatment of high-flow AVMs with large feeders or fistulous connections. Onyx 18 has the ability to travel farther in low-flow situations given its lower viscosity [95].
Once the injection process starts, fluoroscopic visualization of the injection must be attained to ensure anterograde flow of the injected material. Thanks to its nonadhesive nature, the injection and delivery process can be performed slowly, and the injection can be stopped and restarted several times if needed. Initially, a small Onyx cast is allowed to form around the catheter tip (the “plug”). Once created, subsequent injections of Onyx travel into the AVM nidus, and large volumes of nidus can be occluded.
EVOH produces minimal to no inflammatory reaction upon precipitation in tissue in contrast to n-BCA. On the other hand, its solvent DMSO is capable of inducing severe vasospasm and even angionecrosis and rupture if injected too quickly. Slow controlled injection is therefore prudent when using Onyx [96, 97]. Patients also notice a garlic-like taste and a characteristic odor to their breath for several hours to days after Onyx treatment due to DMSO.
EVOH vs. n-BCA
In a prospective, multicenter, randomized trial comparing n-BCA to Onyx for presurgical endovascular embolization of AVM, there was no significant difference between the two agents in terms of AVM volume reduction, amount of surgical blood loss, and surgical resection time. Adverse events between the two agents also showed no statistical significance in the 117 patients’ study [98]. On the other hand, Akin et al., in a swine model experiment, demonstrated easier post-embolization surgical resection of AVMs when Onyx is used compared to n-BCA [99]. This however comes on the expense of a prolonged endovascular procedure time and increased radiation exposure with Onyx [100]. Finally, some evidence suggests that Onyx may be associated with AVM recanalization due to its lower inflammatory-induced angiofibrosis [101]. Which of the two liquid embolic systems to use in which particular clinical situation remains largely an operator preference.
AVM-Associated Aneurysms
There is a strong association between AVMs and intracranial aneurysms resulting from the altered flow dynamics. The reported prevalence of intracranial aneurysms in AVM population varies widely and ranges from 3 % up to 58 % [102–105]. Presence of AVM-related aneurysms significantly increases the risk of hemorrhagic presentations [7, 36, 106].
These aneurysms can simply be classified into intranidal (IN) and extranidal (EN). EN aneurysms can be located in the territory of the AVM (i.e., on a direct feeding artery) or outside this territory in a typical location such as the circle of Willis. Most aneurysms found in hemorrhagic AVM presentations are located intranidally or on a distal feeder close to the AVM nidus, suggesting a higher likelihood that these aneurysms are the source of the bleed [102, 107, 108]. Multiple aneurysms are frequently found as well; however, these are not associated with additional risk versus single aneurysms [102, 104]. No data exists with regard to the size of AVM-related aneurysms at which they pose a critical risk of rupture. However, it is generally agreed that the larger the aneurysm, the higher is its risk of rupture.
The modality as well as timing of treatment for these AVM-associated aneurysms depends on their location as well as presentation. Most of AVM-associated aneurysms (IN and those located within the territory of AVM feeders) are preferentially treated via endovascular coil embolization or liquid embolization, usually prior to treating the AVM itself to avoid any risk of rupture associated with sudden changes in flow dynamics related to AVM treatment [102, 104, 108]. There is some evidence on the other hand to suggest that AVM-associated aneurysms spontaneously regress when the AVM lesion is treated, especially for proximally located aneurysms, and they therefore do not have to be dealt with prior to definite AVM treatment [103, 107]. This is of course unless they are determined to be the source of hemorrhage, in which case urgent treatment of the aneurysm is recommended regardless of its precise location since aneurysmal bleed has a higher early recurrence rate than non-aneurysm-related AVM nidal hemorrhage [102].
Complications and Risks of Endovascular Treatment
Complications of endovascular embolization of AVMs include nonspecific complications such as access site bleeding, contrast allergy, and contrast-related nephrotoxicity. We will focus our discussion here, however, on the specific complications related to endovascular AVM treatment.
The most feared complication of AVM embolization is neurological injury with permanent morbidity or mortality related to ischemia or hemorrhage. Ischemic complications occur when blood clots develop around the delivery and access catheters and wires, when a small artery is mechanically dissected, or when air is introduced accidentally into the system. This is typically minimized with careful manipulation and catheterization of vessels, judicious administration of systemic heparin intravenously during the procedure, and meticulous attention to maintain a closed and continuously flushed access system. More commonly, ischemic injury results from inadvertent embolization or reflux of embolic material into an eloquent vessel [77, 109]. Careful planning of the procedure and proper visualization of target vessels coupled with appropriate choice of embolic agent and its concentration help minimize these potentially catastrophic complications. Pre-embolization provocative testing may also help determine which arterial feeders also serve normal brain function, the so-called en-passage vessels [44, 45, 110].
Hemorrhagic complications can occur either intraoperatively or postoperatively and are related to changes in flow dynamics induced by occlusive embolization of arterial pedicles leading to diversion of flow to areas that cannot withstand the sudden increase in perfusion pressure (normal perfusion pressure breakthrough phenomenon, NPPB) [66]. Hemorrhage can also result from inadvertent embolization of draining veins leading to venous congestion with subsequent hemorrhage [111]. Catheter and wire manipulation can sometimes cause mechanical rupture or perforation of the vessel wall, also precipitating acute hemorrhagic complications. These complications can be minimized by careful visualization of the embolization target with the appropriate choice of the embolization agent concentration to prevent inadvertent venous embolization. Staged embolization over weeks or months may decrease the risk of overwhelming the cerebral autoregulatory mechanism allowing it time to recalibrate and thus preventing NPPB [67, 75, 109].
A number of characteristics are associated with higher rates of morbidity and mortality related to endovascular AVM treatment. These include AVMs with higher Spetzler–Martin grades (grades III–V), those having deep venous drainage, older patients, and those having a normal neurological exam at baseline [67, 109]. Overall, the rate of treatment-related disabling neurological morbidity ranges from 1.6 to 11 % with mortality rates of less than 2 %, with ischemia being a slightly more common cause than hemorrhages [67, 109, 112, 113].
Procedural Considerations
Patient selection: Ruptured AVMs are generally treated with embolization, resection, or a combination of these modalities. The decision to treat unruptured AVMs is more controversial. Young age, low operative risk, high-risk features, and refractory symptoms may argue for aggressive treatment with embolization, resection, radiosurgery, or a multimodality approach. Whether ruptured or unruptured, AVM treatment decisions should be made as part of a multidisciplinary team.
Pre–procedure: A complete diagnostic catheter angiogram must be performed in all cases. This provides information on the location and structure of the nidus, the size and number of its feeders, the venous drainage, and the presence of flow-related stenoses or aneurysms. Digital subtraction angiography at higher frame rates is usually employed to help identify dominant feeders to the AVM and help stratify the most accessible feeders. Pre-embolization microcatheter angiography with or without provocative testing (see Chap. 9) is performed at some centers to select arterial pedicles for embolization but is associated with an elevated risk compared to extracranial catheterization. A three-dimensional image is sometimes helpful to further characterize the lesion and select the optimal imaging angle to utilize during endovascular surgery.
Anesthesia: General anesthesia is the preferred modality in lengthy AVM embolization procedures. It improves image quality through mechanically induced apnea and decreased patient movement. Eliminating the risk of sudden unexpected patient movement during delicate microcatheterization enhances safety. Finally, general anesthesia allows for greater hemodynamic stability and control.
Sheaths: A 6F or larger sheath is generally required. In patients older than 50, we recommend using a femoral sheath length (35 cm or greater) that bypasses any proximal aortoiliac tortuosity. When treating posterior circulation lesions, radial access may be advantageous. In this case, a 6F 11 cm or shorter sheath is recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








