Algorithm for assessment and troubleshooting post-DBS.
On the second day, the patient will have been “off” medication overnight (if the patient has PD) and stimulation will be “on.” The patient will first have stimulation-related amplitude thresholds at each electrode on the DBS lead checked for stimulation-induced adverse effects and stimulation-related benefits. Based on this information, DBS reprogramming is then attempted. Patients can be evaluated using the UPDRS if they have PD, the Fahn–Tolosa–Martin Tremor Rating Scale (TRS) if they have tremor, or the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) if they have dystonia. If the DBS lead is suboptimally positioned and a revision of the DBS lead is being considered, the patient is referred for a full intradisciplinary evaluation to assess if the revision surgery is appropriate. The DBS team members independently evaluate comorbidities, risks, and appropriate targets, and then meet to decide on the best management strategy (medications/device reprogramming versus lead replacement).
Potential problems/issues and solutions encountered with DBS
Troubleshooting surgery-related complications (Table 12.4)
Assessment and management of surgery-related issues and complications can be challenging in the acute time following DBS implantation surgery, mainly because the DBS system often has not been turned on until several weeks after surgery.116 Overall, DBS surgery has had a low mortality rate, with the largest analysis to date involving 4961 patients showing a mortality rate of 0.26% (with 0.15% related to surgical factors).117 In another study, the most frequent reasons for extended hospital stays following DBS implant surgery were mental status change and hemorrhage.118 There was a strong association between lengthened hospital stay and lower MMSE and increased number of MER passes that underscores the importance of careful intradisciplinary patient selection and also careful surgical planning.
| DBS complications | Frequency | |
|---|---|---|
| Surgery-related issues | Intracerebral hemorrhage | 1–5%119–125 |
| Deep cerebral venous hemorrhage/infarction (delayed) | 0.9–2.3%125–127 | |
| Seizure | 0.7–3.1%40,121 | |
| Sterile seroma | – | |
| Pulmonary embolism | 0.4–4.9%128 | |
| Pneumonia | 0.6%128,129 | |
| Perioperative confusion | 1–36%120,130–132 | |
| Suboptimal lead placement | 3.8–12%120,125,133 | |
| Device-related issues | Infection | 3–10%120,124,126,134 |
| Skin erosion | 0.3–6.45%120,121,124,135 | |
| Electrode or wire fracture | 0.94–8.5%120,121,124,136 | |
| Lead migration | 1.7–5.1%120,121,137 | |
| Neurostimulator malfunction | 0.5–18%120,121,136 | |
| Neurostimulator migration | 1–18%120,121,136 | |
| Pain in region of neurostimulator | 11–36%138 | |
| Stimulation-related issues | Vim | |
| Paresthesia | 10%139 | |
| Muscle contractions | – | |
| Dysarthria | 5–25%140–142 | |
| Postural instability/ataxia | 3–7%140,142–145 | |
| STN | ||
| Dyskinesia/hemiballismus | – | |
| Paresthesia | – | |
| Muscle contractions | – | |
| Dysarthria/dysphonia | 4–17%130,146–148 | |
| Gait/postural instability | – | |
| Abnormal eye movements/diplopia | – | |
| Apraxia of eyelid opening | 5%134 | |
| Parkinsonism–hyperpyrexia | Rare149,150 | |
| Neuropsychiatric | ||
| Postoperative confusion | 1–36%120,130–132 | |
| Hypomania | 4–15%131,151 | |
| Anxiety/emotional reactivity | 75%152 | |
| Suicide attempt/suicide | 0.5–2.9%130,153,154 | |
| Depression | 2.9%130,153,154 | |
| Psychosis | 11–16%54 | |
| Cardiovascular reflexes | 48%155 | |
| Sialorrhea | – | |
| Dysphagia | 4–8%148,156–158 | |
| Hypersexuality | 2.4%159 | |
| Weight gain | 6–100%130,148,160 | |
| GPi | ||
| Muscle contractions | – | |
| Dysarthria | Rare131,161 | |
| Visual phenomena | – | |
| Status dystonicus | Rare162 | |
| Other issues | Disease progression | – |
| Poor DBS candidate | – | |
| Lack of an intradisciplinary team to | – | |
| manage patient | – | |
| Unrealistic patient expectation/failure | – | |
| to achieve patient expectations | – | |
| Poor access to DBS programming | ||
| Improper device programming and/or medication adjustment | ||
| Tolerance |
Notes: DBS, deep brain stimulation; GPi, globus pallidus internus; STN, subthalamic nucleus; Vim, ventral intermediate nucleus.
DBS complications that have not yet been investigated or reported with clear frequency ranges are marked “–”.
Intracerebral hemorrhage/subdural hematoma
Intracerebral hemorrhage (ICH) occurs in approximately 1–5% of DBS cases (Figure 12.2).119–122,124,125,163,164 The incidence of ICH seems to be higher in DBS cases performed with microelectrode recording (MER) using multiple electrode passes123,165 and in hypertensive patients, although controversy exists regarding this topic.51 Bleeding may occur several hours or even days after surgery. The risk of bleeding is usually less than 5% per lead implantation,126,166 and around 0.6% of patients will suffer persistent neurological deficits, although the true rate is unclear because several papers have not reported the rate of ongoing neurological deficits.166,167
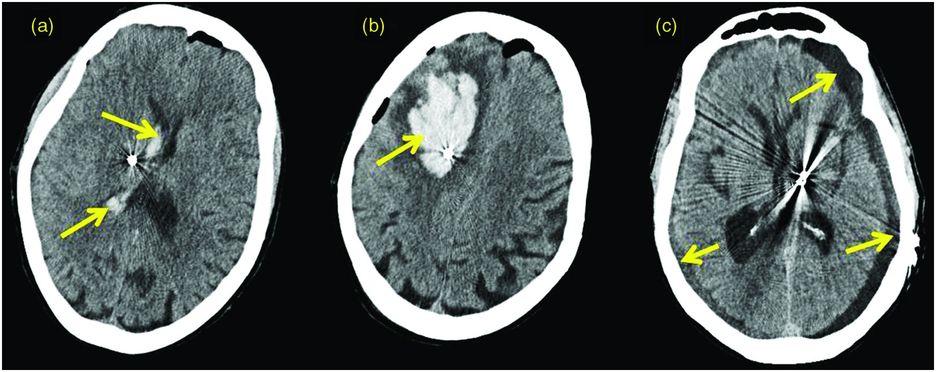
Intracranial hemorrhage after DBS surgery. (a) CT scan showing an intraventricular hemorrhage following DBS lead implant into the right subthalamic nucleus. (b) CT scan showing a large right frontal hemorrhage along the lead tract following DBS lead implant into the subthalamic nucleus (STN). (c) CT scan showing a left STN DBS lead with bilateral panhemispheric subdural effusions, status post-bilateral posterior craniotomies and evacuation of extra-axial fluid.
Prevention
Hemorrhagic complications can potentially be prevented by: (1) planning the most appropriate point of entry into the cranium and by avoiding puncturing superficial veins and sulci near the entry point; (2) controlling blood pressure pre-, intra-, and postoperatively; (3) screening for coagulopathies or recent use of anti-platelet agents before surgery; (4) giving special precaution to MER use in hypertensive patients who require multiple electrode passes;168 and (5) avoiding performing the procedure on patients who have a respiratory condition that would lead to a severe cough or sneeze in an effort to avoid a sudden increase in intracranial pressure and injury during surgery.166
Troubleshooting
It is important to optimize medical management (i.e., maintain airway and ventilation, optimize blood pressure, and optimize intracranial pressure) and neurosurgical preventative interventions. If blood is noted originating from the guide cannula or if deterioration in the level of consciousness occurs and a focal neurological deficit appears, the DBS surgery should be interrupted and a head CT scan should be performed. Sometimes the DBS lead can be expeditiously placed even if hemorrhage occurs in the intraoperative setting; however, this is usually left to the judgment of the neurosurgeon. If the hemorrhage is large, a decompressive craniectomy may be needed to prevent brainstem compression and further complications.169
Delayed deep cerebral venous bleeding/infarction
Cerebral venous thrombosis (CVT) or hemorrhage may occur when a thrombus forms in the large draining veins that enter the dura mater at the site of a burr hole or when a vein is injured or sacrificed during the DBS procedure. Symptoms include severe headache, seizures, change in mental status, and focal neurological deficit. The severity depends on how completely the thrombus occluded the venous territory, causing venous congestion, and on the compensation from collateral vessels.170 Brain MRI venography will show the location of the thrombus in the vein and the dural sinus system.171 A large series of 728 patients reported that cortical/subcortical infarcts occurred in 0.4% of patients.120
Prevention
CVT can be prevented by detailed surgical planning with a gadolinium brain MRI and the placement of burr holes anterior to the coronal suture to minimize venous infarction. Blood screening for certain risk factors such as hypercoagulable states associated with polycythemia vera, antiphospholipid syndrome, protein S and C deficiencies, antithrombin III deficiency, lupus anticoagulant, factor V Leiden, sickle cell, malignancies, etc., prior to surgery may also be helpful in preventing CVT. Preoperative imaging to identify and avoid veins can be useful. Avoiding the sacrifice of veins can in many cases avoid this complication.172
Troubleshooting
CVT can be treated by administering an anticoagulant (unfractionated heparin or low molecular weight heparin) to prevent thrombus spread and increase the possibility of recanalization, although whether this should be done is best decided together with the input of a hematologist. Patients should be maintained at an international normalized ratio (INR) of 2.0–3.5 for at least 3 months. Patients with severe risk factors, such as thrombophilia or antiphospholipid syndrome, may require treatment for a longer time. Anti-thrombotic agents and thrombolytics can also be given to manage CVT. Repeated lumbar puncture or the construction of a lumboperitoneal shunt to reduce intracranial hypertension can be performed to treat headache and papilledema, but these are rarely employed.173 In the rare patient who develops brain herniation and coma, an emergency decompressive hemicraniectomy should be done.174,175 Antiepileptic drugs should be administered in CVT patients with seizure.176
Seizure
The incidence of postoperative seizures after DBS surgery is approximately 0.4–3.1%.120,121,134,163 There are currently no data correlating seizure incidence with the number of microelectrode and/or DBS lead trajectories, although it is likely that greater brain instrumentation, especially if associated with hemorrhage, would place a patient at risk for seizures.
Troubleshooting
When a seizure occurs postoperatively, antiepileptic medications should be administered, usually for a minimum of three to six months postoperatively. The long-term seizure risk is very low.119,122 It is not recommended to administer prophylactic anticonvulsants prior to DBS surgery because these agents may increase medication-related complications and drug–drug interactions, especially in the elderly population.
Sterile seroma
A seroma is a sterile accumulation of serum in a circumscribed location within the tissue. It is caused by an injury during the surgical procedure that may not fully and immediately subside. The remaining serous fluids result in a seroma, with the body usually absorbing the seroma gradually.
Troubleshooting
If the fluid collection does not resolve spontaneously over days or weeks, then drainage by needle puncture may be necessary. If there is any suspicion of an infection, the fluid should be sent for Gram stain, culture, and analysis.
Pulmonary embolism
Pulmonary embolism (PE) occurs at an incidence of 0.4–4.9% after DBS surgery. The mortality rate of non-DBS related PE ranges from 8.6% to as high as 59.4%.128 The risk of PE is increased in PD patients because of their immobility. PE should be suspected in patients who experience sudden onset of dyspnea, tachypnea, pleuritic chest pain, cough, and hemoptysis.
Prevention
Pneumatic leg compressors should be used in patients who are at high risk for PE (for example, those with heart disease, obesity, polycythemia, paralysis of lower extremities, and forced immobilization). Low molecular weight heparin can also be administered to prevent deep vein thrombosis (DVT) and subsequent PE,177 but it should be used only in select cases and preferably with consultation from a hematologist.
Troubleshooting
Low molecular weight heparin should be administered initially and then shifted to warfarin to achieve an INR between 2.0 and 3.0. If another episode of PE occurs while under warfarin treatment, the INR window may be increased to approximately 2.5–3.5.178,179 Additionally, newer anticoagulants not requiring blood monitoring can be considered.
Pneumonia
Postoperative pneumonia is especially common in PD patients with pre-existing swallowing difficulties, which predispose them to aspiration. The incidence rate is approximately 0.6% in the 30 days following DBS surgery.129
Troubleshooting
When pneumonia is suspected, appropriate antibiotics should be administered. Piperacillin/tazobactam or imipenem/cilastatin plus vancomycin are appropriate for many moderate to severe pneumonia cases.180 A culture identifying the organism will provide a better rationale for treatment and aid in determining the choice of antibiotics. Often an infectious disease consult is appropriate.
Postoperative confusion
Postoperative confusion is common following STN DBS surgery. The incidence in reports varies widely, from 1% to 36%.120,130–132,180 Many factors contribute to confusion, including penetration of the frontal lobe, transgression of the ventricular wall,181 long duration of surgery, withdrawal of dopaminergic medications, and pre-existing cognitive deficits. Postoperative confusion is usually transient.
Troubleshooting
If confusion is persistent, a full neurological examination and neuroimaging studies should be performed. Additionally, blood studies, urinalysis, preoperative neuroimaging, and baseline neuropsychological testing should be reviewed to search for pre-existing potential risk factors.
Suboptimal DBS lead placement (Figure 12.3)
Suboptimal DBS lead placement is one of the most common causes of DBS failure.1 Factors that may result in a misplaced lead include poor surgical technique, head movement or frame shift, misinterpretation of MER data, and brain shift.125,136 The incidence varies from 1.2% to 12% of patients.120,121,125,133,136,163
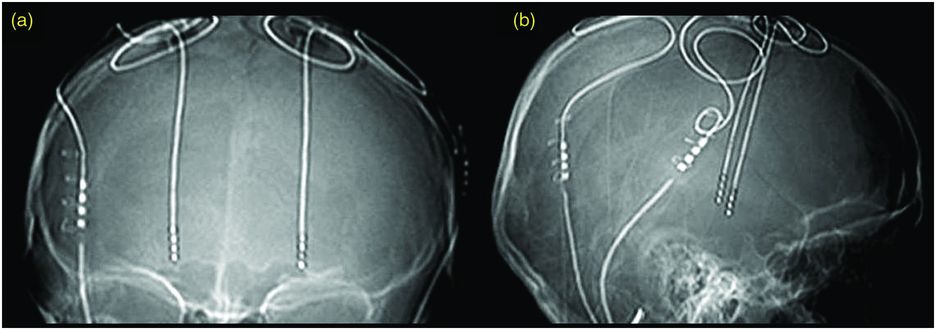
Suboptimal DBS lead location. Plain x-ray, AP (a) and lateral (b), showing suboptimal left DBS lead placement following STN DBS surgery. The best imaging technique to identify lead location is MRI, if available.
Prevention
Suboptimal lead placement can be minimized by the following strategy: (1) improving surgical technique; (2) confirming DBS lead position during surgery by intraoperative fluoroscopy before and after the lead has been locked by a securing device, and by obtaining a head CT scan/brain MRI postoperatively to confirm the position of the lead;116 (3) minimizing traction on the brain while fixing the lead.
Troubleshooting
1. Test stimulation amplitude thresholds that produce benefits and adverse effects for each electrode on the DBS lead and attempt programming at one month after surgery.
2. Evaluate the lead location using MRI (Figure 12.4), following the safety guidelines of the device manufacturer (a CT scan can be done if there are contraindications to an MRI) and determine the anatomical three-dimensional position of each electrode on the DBS lead.
3. Gather and review all data (clinical assessment, stimulation-related adverse effects, benefits derived from stimulation, and lead position) and decide whether there is an electrode that can be used to deliver stimulation and provide benefit.
If the benefit of device programming cannot be sustained for more than hours or a few days, or if stimulation-induced adverse effects occur at low stimulation thresholds, a suboptimally located lead should be suspected and replacement of the intracranial lead considered.116
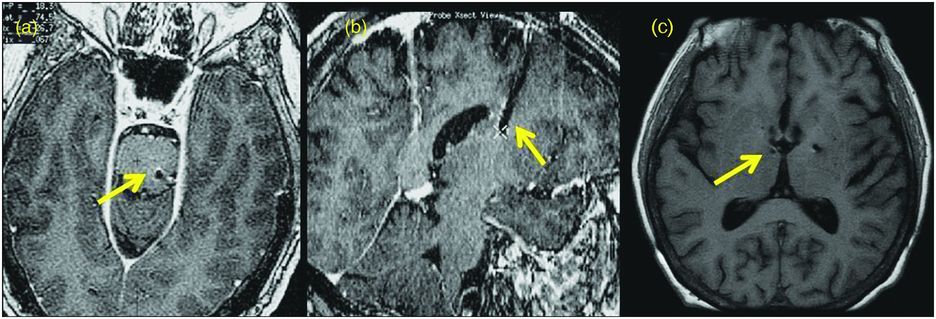
A suboptimally placed DBS lead. (a) Brain MRI in the axial plane showing that the left DBS lead position is too deep beneath the pontomedullary junction. (b) Brain MRI in the sagittal plane demonstrating that the right DBS lead position is too shallow within the periventricular white matter of the centrum semiovale following STN DBS surgery. (c) Brain MRI in the axial plane revealing that the right DBS lead was placed too far medial after GPi DBS surgery.
Management of device-related issues
Infection (Figure 12.5)
Infection is the most common device-related surgical complication. The incidence ranges from 3% to 10% in most series.120,121,124,126,134,163,164,182,183 In cases of high infection rates (12–15.1%),124,184 the hospital infectious disease committee should be called in to investigate possible sources within the hospital and its employees. Most often, infection involves the neurostimulator in the pectoral region and not the DBS lead itself. In general, infected device components need to be removed and antibiotic treatment initiated. Treatment with antibiotics alone, without device removal, is not likely to be effective. If the infection involves the DBS lead itself, leaving it in place can potentially result in a severe intracranial infection, such as a subdural empyema or brain abscess.
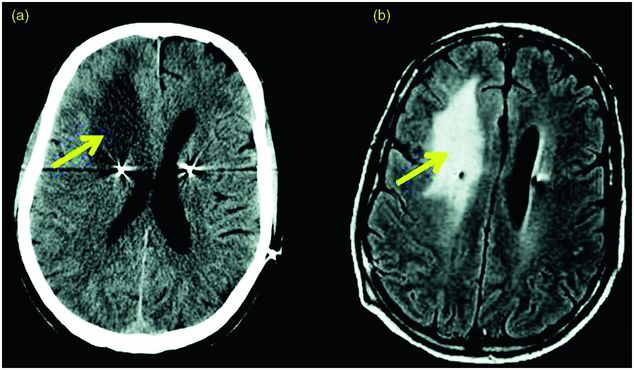
DBS-related infection. (a) CT scan in the axial plane showing bilateral STN DBS lead implantation and a hypodensity in the right frontal area (vasogenic edema), which has resulted in a right-to-left subfalcine shift. (b) MRI scan in the axial plane showing fronto-parietal edema due to an infectious process.
Prevention
Several recommendations have been made for reducing the postoperative infection rate. These include: (1) using strict aseptic surgical methods (e.g., washing the surgical skin site with chlorhexidine);185 (2) improving surgical technique (e.g., a curvilinear incision is preferable to evade crossing of the lead anchoring device); (3) reducing surgical time; and (4) using antibiotics (cefazolin).136,183 The first dose of cefazolin should be given before surgery; the second and third doses after surgery. If the patient is sensitive to cefazolin sodium, one dose of vancomycin hydrochloride can be administered before surgery.136 The role of shaving off hair to prevent infection is still controversial,186 and many centers such as ours perform hair-sparing operations with a low infection rate. In case hair needs to be shaved, an electric clipper is preferable to a razor in order to lower the infection rate.187
Troubleshooting (Figure 12.6)
If the infection is superficial and mainly a localized skin infection of the surgical wound, it may be treated with intravenous or even oral antibiotics with adequate tissue penetration. A close clinical follow-up visit must be performed to evaluate the infection. If the infection does not improve with the antibiotics, the extension and neurostimulator should be removed. Repeat the culture and administer the appropriate antibiotics based on the findings of the culture (the antibiotic course should be for six to eight weeks). Implantation of new hardware can be performed once the infection has cleared.
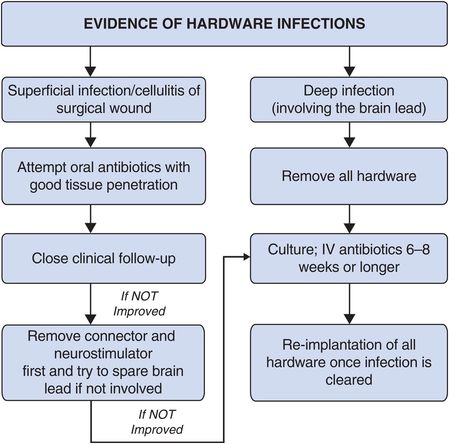
Algorithm for troubleshooting deep brain stimulation infections.
If the infection is deep and involves purulence around the brain lead, it must be treated by immediate removal of the hardware. Perform culture studies and administer a sufficient course of antibiotics (usually at least six to eight weeks) prior to replacement of hardware. In severe cases of deep, purulent infection around the brain lead, it is often prudent to consult an infectious disease specialist.
Skin erosion
Skin erosion occurs mostly at the connector site that joins the DBS lead to the extension. It is usually a late-occurring complication, with an incidence of 0.6–6.5%.120,124,135,164 The original connector used in the past was bulkier, which may have contributed to this complication. Use of the newer connectors, combined with proper placement under the scalp and meticulous galea closure work, will decrease the risk of skin erosion.126,136 Larger neurostimulators may also be more likely to cause skin erosions. Skin erosions may also occur because of extremely tight suturing over the device, or in those elderly patients with thin, frail skin.
Prevention
Erosions of the skin above the neurostimulator can be prevented by implanting the lead connector or neurostimulator into a deeper tissue plane and using one tunnel for each neurostimulator.138 Skin above the DBS system should be checked at each visit so that problems that may cause skin erosion can be identified and treated early.
Troubleshooting
If the erosion occurs over the connector, it can be relocated into a trough drilled in the skull. If the erosion occurs over the lead and cap, a complete revision of the device is required, moving it to a deeper plane if necessary.124,188 If the erosion occurs over the burr hole and no infection was found, a skin revision may be sufficient. Sometimes a revision is needed to replace the burr-hole ring and cap assembly using bone cement or methacrylate and a ligature or plate to lessen pressure on the incision and help closure of the wound.
DBS lead or wire break (Figure 12.7)
Most lead or wire breaks occur between the extension cable and the DBS brain lead, which is usually located below the mastoid process.137,163,189–192 Head turning can mobilize the DBS lead connector and can break the system circuit. The incidence of lead or wire break has been reported in various series to range between 0.94% and 8.5%.120,163,190–192 There may be a difference in lead fracture rates in different disease targets,190 although this is unclear.193 In addition, in a large retrospective review involving 531 procedures, Servello et al. showed a statistically significant association with infectious complications in the Tourette syndrome subgroup, again suggesting possible differences between diseases or disease targets (although the etiology underpinning the differences is unknown).194 If a difference exists, one potential explanation would be the increased involvement of violent head movements in ET, dystonia, or Tourette patients, although there could be inherent differences in the different targets. This could also skew reported rates of complications based on the composition of studies. Some patients may feel an “electric shock” sensation that is worsened by pressing on the area of the lead. Impulse control disorders such as trichotillomania195 and twiddler syndrome have been reported to cause DBS lead or wire fractures.196–199 In twiddler syndrome, the patient deliberately or subconsciously spins the neurostimulator in the chest region. When the patient spins the neurostimulator, the lead is shortened and an open circuit occurs, causing an electrical sensation in the patient’s body. Alternatively, this may also result in a lead fracture.
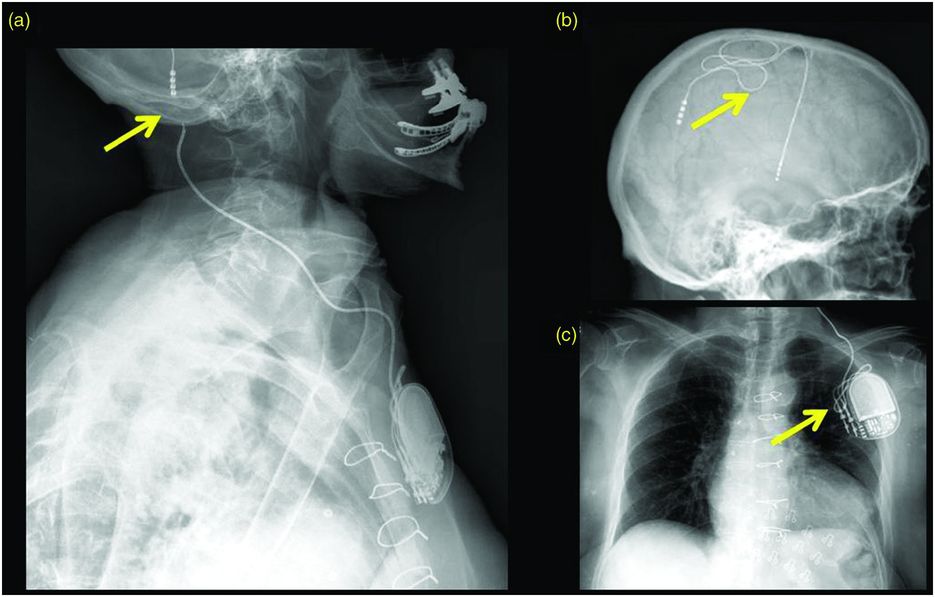
Example of a DBS lead fracture. Plain x-ray film series through the skull, neck, and chest of a “twiddler” patient demonstrating a DBS extension coursing through the neck and terminating in the left chest wall. (a) There is discontinuity in the wiring at the level of the left mastoid process. (b, c) The DBS lead and extension are coiled in the extracranial soft tissues; this is usually a result of ongoing manual manipulation of the neurostimulator.
Prevention
To prevent lead fracture, the DBS lead connector should be mounted on the skull in the parietal subgalea region and not in the neck.
Troubleshooting
Device electrode impedances should be checked on every clinic visit. A high impedance usually indicates a break or open circuit. A low impedance usually indicates a short circuit.200 In a twiddler patient, it is necessary to create a new suitably sized pocket under the pectoralis muscle fascia (usually on the contralateral side), suture the device to the muscle, and use a Parsonnet pouch to prevent an electrode or wire break.197,201
Lead migration (Figure 12.8)
Lead migration is a rare event, occurring in 0.5% in a large series of 728 patients,120 although some have reported rates higher than 4% (reviewed by Boviatsis et al.163). Migration usually occurs when fixation of the lead to the skull is inadequate.136,189 In dystonia, the lead may possibly shift downward due to head movement, dragging the extension cable and neurostimulator upward.202 Another potential cause of lead migration is skull growth in children over time, and in this case the lead migrates dorsally.116
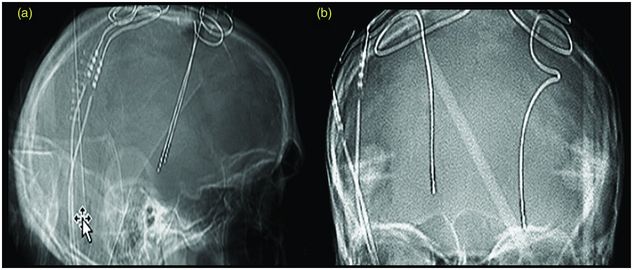
Lead migration in a patient with GPi DBS. Plain skull x-ray lateral (a) and AP (b) showing lead migration status post GPi DBS in a patient with generalized torsion dystonia who developed worsening of symptoms that were previously controlled by DBS. The left lead had moved 10.4 mm downward and the right lead had moved 4.7 mm upward.
Prevention
Lead migration can be prevented by carefully securing the DBS lead and anchoring the lead using the burr-hole ring and cap in the subgaleal space. If using a titanium microplate,203 be sure not to crush the lead under it. After the lead is anchored, make a wide lead wire loop around the cap to reduce traction when there is pulling of the system.202 Perform intraoperative fluoroscopy to ensure that the lead is in the correct location before closing the scalp incision.
Troubleshooting
1. Verify lead position by performing serial postoperative scans, especially in patients with waning clinical benefit.
2. Determine whether there is movement of the lead(s).
3. If there is dorsal displacement, compensate with reprogramming (using the deepest/ventral electrode for reprogramming).
4. Consider another surgery for repositioning and replacement of the DBS lead if clinical benefit cannot be recaptured.
Battery (neurostimulator) life and failure
Battery (neurostimulator) life is dependent on inherent battery voltage, stimulator parameters and usage, battery chemistry, impedance fluctuations, interpolation errors, usage patterns, and self-discharge. Estimators of battery life typically employ calculations based on initial battery capacity and current drain. The main contributor to current drain is therapeutic impedance.204,205 In addition, battery life estimation varies by neurostimulator model and requires device-specific algorithms. Battery replacement algorithms should incorporate clinical status, as reduced symptom control can occur toward the end of battery service life.204,205
Prevention
At each clinical visit an estimation of battery service longevity should be performed and clinical symptom worsening should be documented. If clinical symptoms are worsening toward the end of battery life, or alternatively if the patient is treated with high levels of stimulation, one must consider that worsening of symptoms could be due to battery depletion. The battery service life can be generally estimated by consulting technical support provided by the device manufacturer or it can be estimated by using the web-based calculator or iPhone app from the University of Florida: http://mdc.mbi.ufl.edu/surgery/dbs-battery-estimator.
Neurostimulator malfunction (Figure 12.9)
Most neurostimulator malfunctions have occurred with earlier neurostimulator models and are now uncommon. Patients complained of shocking sensations or intermittent stimulation around the neurostimulator site.136
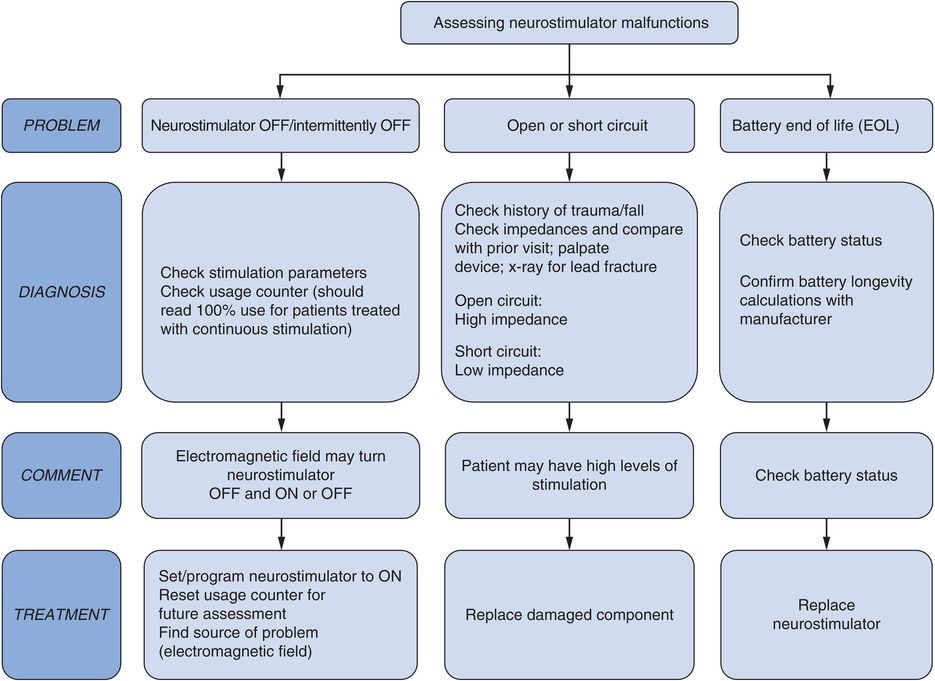
Assessment of neurostimulator malfunction.
Prevention
During follow-up visits, the clinician will need to verify proper functioning of the device.
Neurostimulator migration
Neurostimulator migration results mainly from a change in the subcutaneous position of the device and may be due to inadequate securing of the device during implantation or due to obesity of the patient.120 Compulsive behavior (twiddler syndrome) may increase the risk of device migration and lead or extension fracture.199
Prevention
Migration can be prevented in many cases by suturing the neurostimulator under the underlying fascia below the pectoralis muscle and by using a polyester pouch.
Troubleshooting
Verify the diagnosis by visualizing the neurostimulator with a “shunt series” x-ray examination. Problems uncovered sometimes require management by surgical revision.
Pain/discomfort over the neurostimulator
Pain/discomfort over the neurostimulator is mostly found in thin patients whose lead-extension path has one tissue tunnel for both lead extensions (36%), rather than in those with a single tunnel for each lead extension (11%).138
Prevention
Consider implantation of the neurostimulator in the abdominal region for thin patients.138 In our own experience, we have not found this approach of abdominal implantation to be helpful, and we usually opt for a more conservative approach that may also include exercises of the neck region.
Troubleshooting
Capsaicin topical cream 0.025% or 0.075%119 can be applied 4 times a day over the neurostimulator region to reduce pain. Neurostimulator repositioning may be necessary if capsaicin treatment fails to relieve the pain/discomfort.
Management of stimulation-related issues
Stimulation-related issues usually result from the spread of electrical current into brain regions other than the region intended for stimulation and from suboptimal lead placement. The clinician programming the DBS system should keep in mind that stimulation using an appropriately placed DBS lead should not produce stimulation-induced adverse effects at low to medium levels of stimulation, but may (depending on DBS target) be expected to produce stimulation-related adverse effects at medium to high levels of stimulation. For example, in DBS of the thalamic Vim nucleus, stimulation can produce paresthesia, dysarthria, muscle contraction, and postural instability, depending on the current density delivered. With DBS of the STN, patients may experience dysarthria, tonic muscle contraction, dyskinesia, paresthesia, and diplopia. In response to DBS of the GPi, there may be hypophonia, dysarthria, tonic muscle contraction, paresthesia, and visual phosphenes. Poorly placed DBS leads typically result in adverse effects at stimulation levels lower than that required for clinical benefit. Stimulation-related adverse effects are reversible and may be avoided in many cases by adjusting stimulation parameters.
Stimulation using an appropriately placed DBS lead should not produce stimulation-induced adverse effects at low to medium levels of stimulation, but is expected to produce stimulation-related adverse effects at medium to high levels of stimulation.
A poorly placed DBS lead may either produce stimulation-related adverse effects at relatively low levels of stimulation or may not produce any effects, beneficial or adverse, at relatively high levels of stimulation.
Troubleshooting
Test stimulation through each electrode on the DBS lead is frequently performed in a unipolar configuration, with a gradual escalation of stimulation amplitude applied; amplitude thresholds for the emergence of adverse effects should be explored and documented. If stimulation-related adverse effects occur outside the expected range (that is, at relatively low levels of stimulation) or not at all, imaging should be performed (preferably with a brain MRI following the safety precautions provided by the device manufacturer or with CT scan fused to the preoperative MRI). This procedure will usually help to verify if the DBS lead is in an acceptable position, and it can help in choosing the best electrode on the DBS lead for therapeutic stimulation. If the DBS lead is found to be in a suboptimal location, stereotactic surgical repositioning, possibly with physiological guidance, may be required.
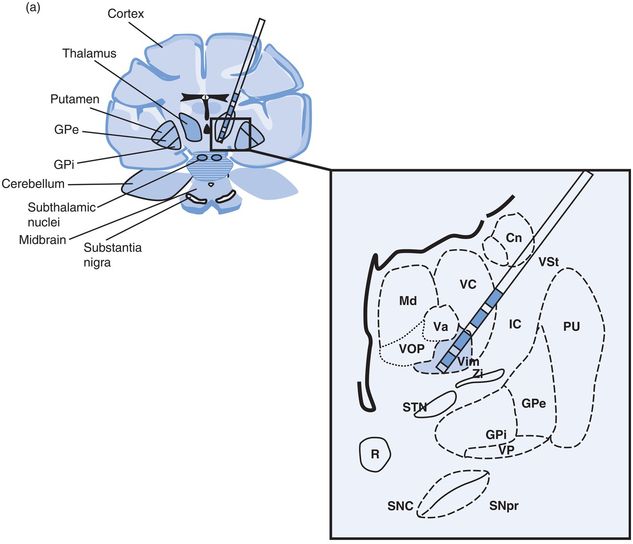
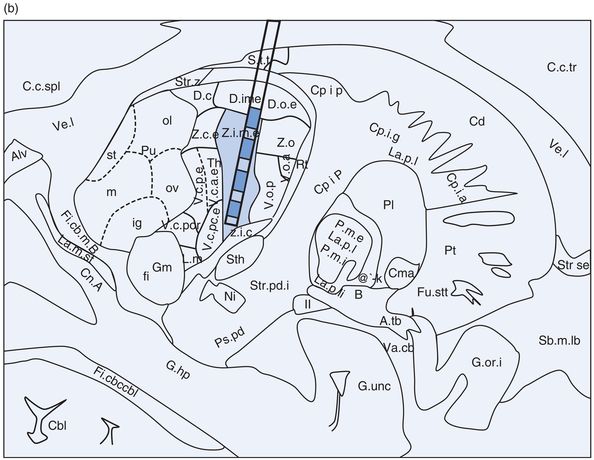
Problems specific to thalamic Vim DBS (Figure 12.10)
Thalamic ventral intermediate (Vim) nucleus DBS lead location in (a) coronal and (b) sagittal planes.
Paresthesia
Paresthesia is defined as an abnormal sensation of the skin, such as numbness, tingling, pricking, or burning. As stimulation level is increased, transient paresthesia may occur, usually disappearing within a few seconds. At higher levels of stimulation, up to 10% of patients will experience persistent paresthesia.139 This effect is due to electrical current from the active electrode(s) stimulating the ventralis caudalis (Vc) nucleus of the thalamus or alternatively the lemniscal fibers.206
Troubleshooting
Persistence of paresthesia can be managed by reprogramming the device (lowering the amplitude or pulse width, or by changing the electrode configuration). If reprogramming fails to provide the patient with adequate tremor control in the absence of persistent, bothersome paresthesia, repositioning of the DBS lead should be considered.144
Muscle contraction
Muscle contraction occurs when stimulation spreads into the internal capsule.207 The contracting muscles usually correspond to the region of the capsular homunculus.
Troubleshooting
Muscle contraction can be diminished by reducing the stimulation level or by changing the electrode configuration.208
If reprogramming fails to provide the patient with adequate tremor control in the absence of persistent muscle contraction, repositioning of the DBS lead should be considered.144
Dysarthria
Approximately 5–25% of patients will experience stimulation-induced dysarthria with bilateral Vim DBS.140–142 Dysarthria may be caused by stimulation current spread to the internal capsule/corticobulbar fibers, and this may commonly occur with Vim because the Vim is anatomically close to internal capsule.208,209
Patients with dysarthria following Vim DBS should be examined in two conditions: “on” stimulation and “off” stimulation. Dysarthria due to stimulation will get worse when stimulation is “on.” This can sometimes be treated by decreasing the stimulation parameters or by changing the configuration of electrodes, especially to a bipolar montage. If the DBS lead was placed too lateral, the stimulation will influence corticobulbar fibers and cause dysarthria at a level of stimulation that is insufficient to control tremor. This problem can sometimes be managed by repositioning the DBS lead, although many patients opt to accept the presence of some dysarthria in exchange for tremor control (and can use their patient programmer to adjust stimulation levels to optimize either tremor control or clarity of speech, depending on their circumstances). Dysarthria can also occur with other DBS targets.
Postural instability/ataxia (Figure 12.11)
Postural instability and other problems with balance have been reported following Vim DBS, with an incidence of approximately 3–7%.144 These problems seem to be associated with bilateral stimulation.210 The symptoms may reverse in some cases with stimulation parameter changes,140–143,145,209 usually reductions in amplitude or pulse width, or switching to bipolar configuration. These issues may also emerge with other targets and diseases.
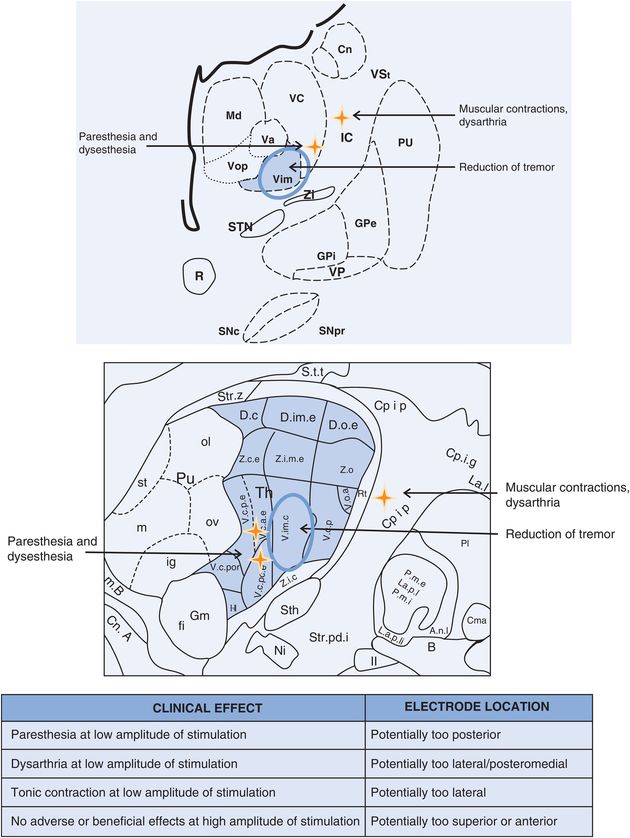
Vim DBS: stimulation-induced effects. Cn, caudate nucleus; GPe, globus pallidus externus; GPi, globus pallidus internus; IC, internal capsule; Md, nucleus mediodorsal thalamus; Pu, putamen; R, red nucleus; SNc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; STN, subthalamic nucleus; Va, nucleus ventral anterior thalamus; Vc, nucleus ventrocaudal thalamus; Vim, nucleus ventral intermediate thalamus; VP, visual pathway/optic tract; Vop, nucleus ventral oral posterior thalamus; VSt, ventral striatum; Zi, zona incerta.
Problems specific to STN DBS (Figures 12.12 and 12.13)
STN DBS lead location in (a) coronal and (b) sagittal planes. Cn, caudate nucleus; GPe, globus pallidus externus; GPi, globus pallidus internus; IC, internal capsule; Md, nucleus mediodorsal thalamus; Pu, putamen; R, red nucleus; SNc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; STN, subthalamic nucleus; Va, nucleus ventral anterior thalamus; Vc, nucleus ventrocaudal thalamus; Vim, nucleus ventral intermediate thalamus; VP, visual pathway/optic tract; Vop, nucleus ventral oral posterior thalamus; VSt, ventral striatum; Zi, zona incerta.
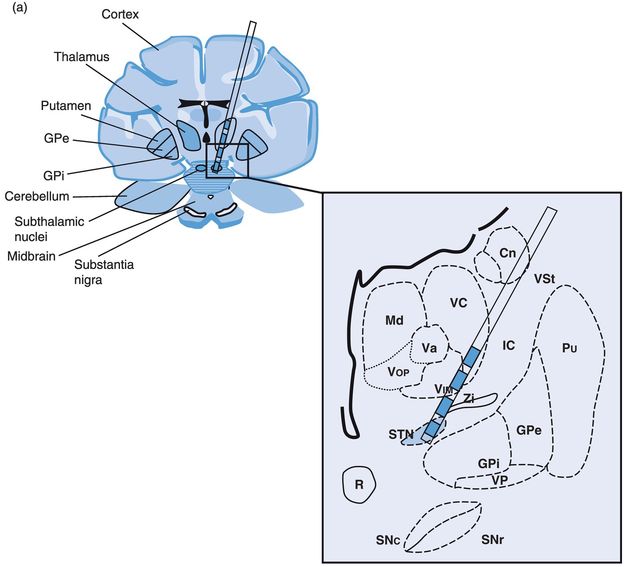
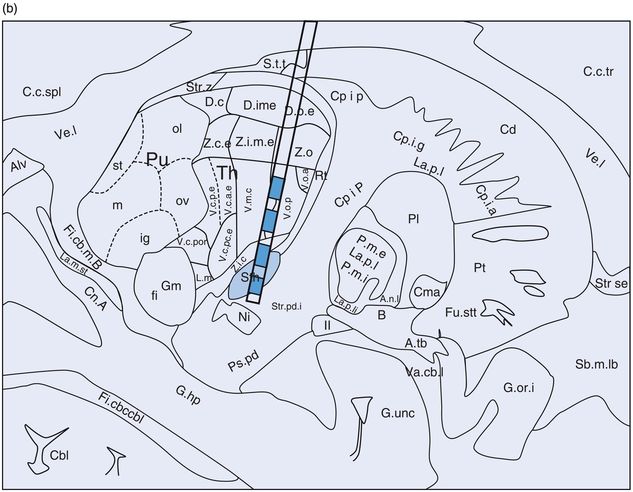
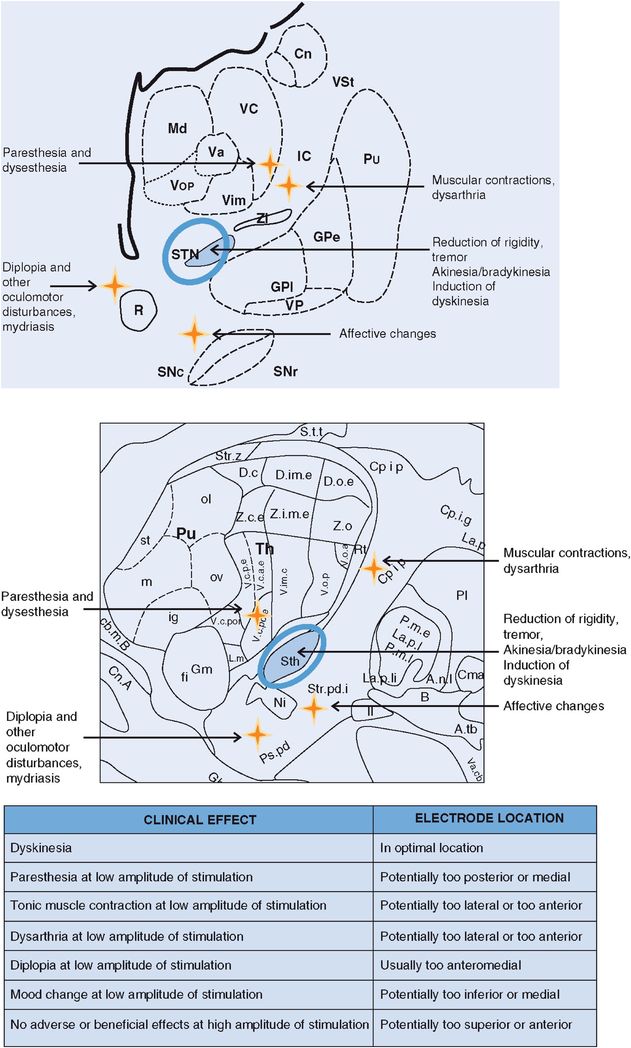
STN DBS: stimulation-induced effects. Cn, caudate nucleus; GPe, globus pallidus externus; GPi, globus pallidus internus; IC, internal capsule; Md, nucleus mediodorsal thalamus; Pu, putamen; R, red nucleus; SNc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; STN, subthalamic nucleus; Va, nucleus ventral anterior thalamus; Vc, nucleus ventrocaudal thalamus; Vim, nucleus ventral intermediate thalamus; VP, visual pathway/optic tract; Vop, nucleus ventral oral posterior thalamus; VSt, ventral striatum; Zi, zona incerta.
Dyskinesia/hemiballismus
Stimulation-induced dyskinesia with STN DBS appears to be a sign of a well-located DBS lead. Dyskinesia may occur minutes to hours after stimulation is initiated or increased, requiring that the clinician programmer be patient while evaluating the subject and, if possible, observe them after some time has passed. Controlling dyskinesia is best accomplished by balancing stimulation parameters and dopaminergic medication.144 Often, reduction of medications is the most effective treatment. However, there are cases of “brittle” dyskinesia after STN DBS and in these cases a GPi DBS could be a possible rescue strategy if aggressive programming and medication changes cannot alleviate the issue.211,212
Paresthesia
Paresthesia usually occurs as a result of current spread to the medial lemniscus, which passes ventroposteriorly to the STN. In most instances, these paresthesias are transient, disappearing rapidly. Persistent paresthesia may indicate that the DBS lead is located too deep, posterior, and medial. Adjusting the stimulation parameters or electrode configuration usually resolves this problem.144
Muscle contraction
Muscle contraction in STN DBS may be due to current spread into the internal capsule.144,207 The muscles that contract correspond to the region of the capsular homunculus.208
Dysarthria/dysphonia/hypophonia
The prevalence of impaired speech after STN DBS ranges from 4% to 17%,130,146–148 and this impairment typically occurs after bilateral STN DBS. Most studies assume that speech impairment is due to current spread to internal capsule and corticobulbar fibers,208,209,213 but it may be also be related to the progression of PD.
Troubleshooting
For speech problems after STN DBS, adjust and optimize stimulation parameters and consider the effects of left versus right stimulation and the effects of medications. Speech therapy may be helpful, and in severe situations patients may need to temporarily turn off one stimulator (usually the left) when speaking.214–216 If the issue does not resolve when the DBS is switched to an off position it may indicate disease progression or a surgical implantation effect.
Gait impairment/postural instability
Gait impairment and postural instability are often refractory to DBS treatment and may actually worsen post-DBS. Patients with Parkinson’s disease who have improvements in gait impairment and postural instability with levodopa treatment are more likely to have benefit, but this is unpredictable. Patients who did have improvement in axial symptoms with DBS tend not to have sustained benefits.217 It remains unknown whether these symptoms are amenable to targeting of other structures, such as the PPN.
Troubleshooting
Balancing the stimulation parameters, dopaminergic medication, and occupational/physiotherapy may be helpful in improving gait and postural impairment in patients with PD. In the case of stimulation-induced postural instability with truncal ataxia, suboptimal lead location may be the cause, and this may result in current spread to the red nucleus or cerebellar fibers.218 This complication can be managed by revision of the DBS lead.
Abnormal eye movements/diplopia
Double vision (diplopia) is usually due to stimulation of supranuclear oculomotor fibers below or medial to the STN, resulting in disconjugate eye movement. Patients may experience dizziness when viewing images displaced horizontally, vertically, or diagonally.
Troubleshooting
Most of the disconjugate eye movements after DBS are transient, due to habituation. If symptoms persist after the stimulation parameters and electrode configuration are optimized, revision of the lead may be required.144
Apraxia of eyelid opening
Apraxia of eyelid opening (ALO) is a condition where patients who have otherwise normal eyelids have difficulty opening the eyelids and experience involuntary closure of the eyes. It most commonly occurs following STN stimulation in patients who obtain good motor symptom improvement with the DBS.208,219 This adverse event is relatively infrequent, occurring with an incidence of approximately 5%.134,164 The mechanism of ALO is not well understood, and it can occur with low energy parameters. Adjustment of the stimulation parameters may or may not be of benefit.
Troubleshooting
Most ALO patients are successfully treated by injection of botulinum toxin into eyelid regions,219 or by reprogramming the device to deliver stimulation using a different electrode configuration. When botulinum toxin is used, the injections are usually administered to the pars palpebralis of the orbicularis oculi muscle and need to be repeated every three to six months. These injections carry minimal risk of transient adverse effects such as hematoma, double vision, dryness of the eyes, or ptosis.116
Parkinsonism hyperpyrexia syndrome
There have been rare case reports in which parkinsonism hyperpyrexia syndrome, a neuroleptic malignant-like state, has been reported with abrupt withdrawal of STN stimulation. There have only been scattered case reports associated with STN stimulation, and it is not known whether this is a possibility with sudden cessation of GPi DBS.149,150
Troubleshooting
In a PD patient with DBS implantation who presents to the ICU with features of a neuroleptic malignant-like syndrome, dopamine withdrawal should be suspected, but sudden DBS failure should also be part of the differential. Using gradual stimulation reductions when necessary has been proposed to prevent this complication, though there is little data on its management.149,150
Neuropsychiatric complications (Figure 12.14)
Neuropsychiatric complications vary greatly in their manifestations. These complications have been increasingly documented following DBS treatment. Some of the more common neuropsychiatric conditions that have been observed following DBS surgery and/or with DBS treatment are transient confusion, anxiety, apathy, hypomania, emotional instability, depression, suicidal tendencies, psychosis/hallucination, and compulsive or impulsive behavior.220 A psychiatric referral may be needed to assist in evaluation and treatment of the patient.221–223
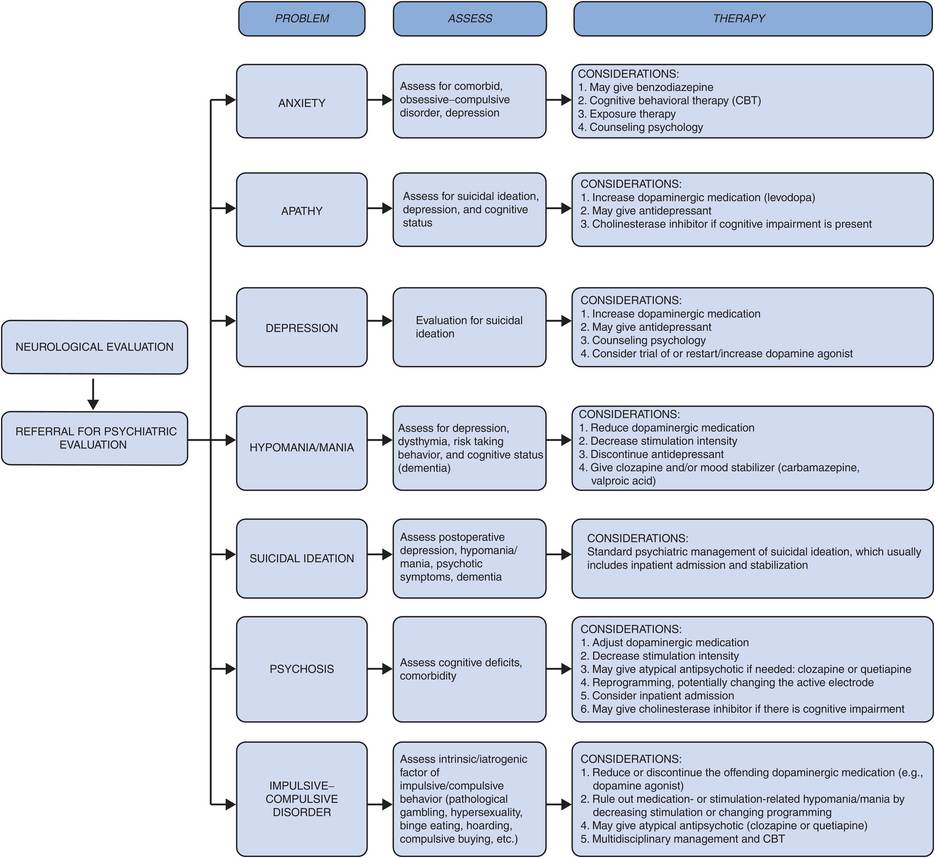
Management of neuropsychiatric complications.
Anxiety or emotional reactivity is the most common nonmotor symptom in PD. It occurs in 75% of patients pre- and postoperatively.152,154 Anxiety frequently occurs in patients who suffer from depression, panic attacks, and phobia. A trial of a benzodiazepine, cognitive behavioral therapy (CBT), and exposure therapy could be useful, depending on the individual situation. There are also now counseling and online programs that can be useful to address anxiety.
Transient confusion has been reported to occur in 1–36% of patients in the immediate postoperative phase.130–132,224 Because it usually improves with time, no immediate treatment is needed.
Apathy also occurs, with a reported approximate incidence of 4–25% after STN DBS therapy.130,225 It is sometimes precipitated by depression associated with the reduction of dopaminergic medication that may occur when motor symptoms are improved by DBS; increasing the dose of levodopa or a dopamine agonist may therefore help to alleviate the apathy. The patient should be assessed for depression or mood disorder and given antidepressants if needed. Depression itself occurs in 1.5–25% of patients following STN DBS treatment.130,131,151,157 It can be caused by many factors, such as the natural history of PD itself, dopaminergic medication withdrawal, current spread to the medial limbic system, and psychosocial factors such as unrealistic patient expectations and poor family support. This manifestation can be managed by treating the underlying cause of the depression by adjusting levodopa or dopaminergic medication, giving antidepressant medications, modifying the stimulation parameters, and by consulting with a psychiatrist.220
Hypomania occurs in 4–15% of patients post-DBS surgery.131,151 Treating postoperative hypomania can be performed by adjusting the dose of levodopa and by administering a trial of antidepressants and mood stabilizers. A cholinesterase inhibitor may be given if cognitive impairment is present, and CBT may also be beneficial. In some cases, changing the active stimulation electrode used for treatment has improved the situation.
Prevalence of suicide attempts after DBS varies from 0.5% to 2.9%.46,153,154,220 Depression, hypomania/mania, and psychotic symptoms should be evaluated, and patients exhibiting the behaviors and symptoms of these neuropsychiatric conditions in most cases should be admitted to the hospital and also referred to a psychiatrist for proper outpatient management.
Psychosis (11%) and transient hallucination (16%) have been reported in association with DBS.54 The physician should consider admitting the patient to adjust dopaminergic medication and possibly also changing stimulation settings. An atypical antipsychotic may be given to treat psychosis. Most clinicians use clozapine or quetiapine, which tend to not worsen PD motor symptoms.
Impulsive–compulsive disorder (ICD, e.g., pathological gambling, hypersexuality, binge eating, compulsive shopping, and hoarding) may be present or be precipitated by DBS.226,227 There may also be an underlying dopamine dysregulation that can be unmasked or worsened by DBS. It is important that patients be monitored pre- and postoperatively for the possibility of emergence of ICD and dopamine dysregulation syndrome (DDS). These cases can be managed by decreasing or discontinuing dopaminergic medication (particularly dopamine agonists), adjusting DBS parameters, treating with atypical antipsychotics, and also using drugs such as valproic acid or naltrexone.211,228
Cardiovascular reflexes
Based on the pathophysiological circuitry model of basal ganglia function in PD, there is an increased excitatory output from the STN that may possibly result in an increased inhibitory output from the thalamus.229 This is hypothesized to reduce the compensatory tachycardic response to postural changes required to prevent symptomatic orthostasis,230 and the effects of DBS have been implicated in autonomic function.
An observation by Sauleau et al.,231 who directly monitored patients for intraoperative autonomic changes following STN DBS for PD, revealed that 88% of patients had tachycardia within seconds after stimulation, followed by a hypertensive interval after approximately 1 minute. This effect was temporary, and the autonomic system returned to normal a few minutes following discontinuation of DBS.155 Some studies suggest that tachycardia may persist for six months after DBS treatment with high-frequency stimulation232 and disappear after one year.155 The long-term clinical significance of transient tachycardia and hypertension is still unknown.
Troubleshooting
Close monitoring of blood pressure and pulse rate after DBS can be important in select cases. Fluctuations in blood pressure or cardiovascular function can be managed medically. Antihypertensive medications may need to be reduced in dose, and overall hydration may need to be increased.
Bladder function
Nonmotor symptoms are highly prevalent in advanced PD patients, with the most common in large studies being nocturia, urinary urgency, and constipation. Although there is a large amount of data on the motor benefits of DBS in Parkinson disease, there are very few data on the effects of DBS on these nonmotor symptoms. Most studies have employed a questionnaire to assess nonmotor symptoms pre-DBS and post-DBS.233,234 There have been conflicting reports on the effects of DBS on urinary function. Early studies have shown some benefits in urinary symptoms that were measurable by urodynamic studies with STN DBS.235 In a study by Winge et al., patients who underwent DBS showed improvements in symptoms of nocturia without changes in bladder emptying.236 Subsequent questionnaire-based studies reported no difference in urinary symptoms after DBS.233,234 There have been isolated case reports of urinary retention following STN DBS in those with previously normal bladder function.237 Taken together, the data are very limited, although some patients may report a benefit in urinary symptoms and a minority may experience worsening.
Troubleshooting
Patients with urinary complaints may require urodynamic studies and should be managed in collaboration with a urologist who can provide recommendations regarding particular urinary dysfunction issues. Urinary retention post-DBS is a medical emergency and would require catheterization and discontinuation of anticholinergics, and may require a long-term suprapubic catheter.237 Post-DBS urinary retention in men could be related to prostatic hypertrophy.
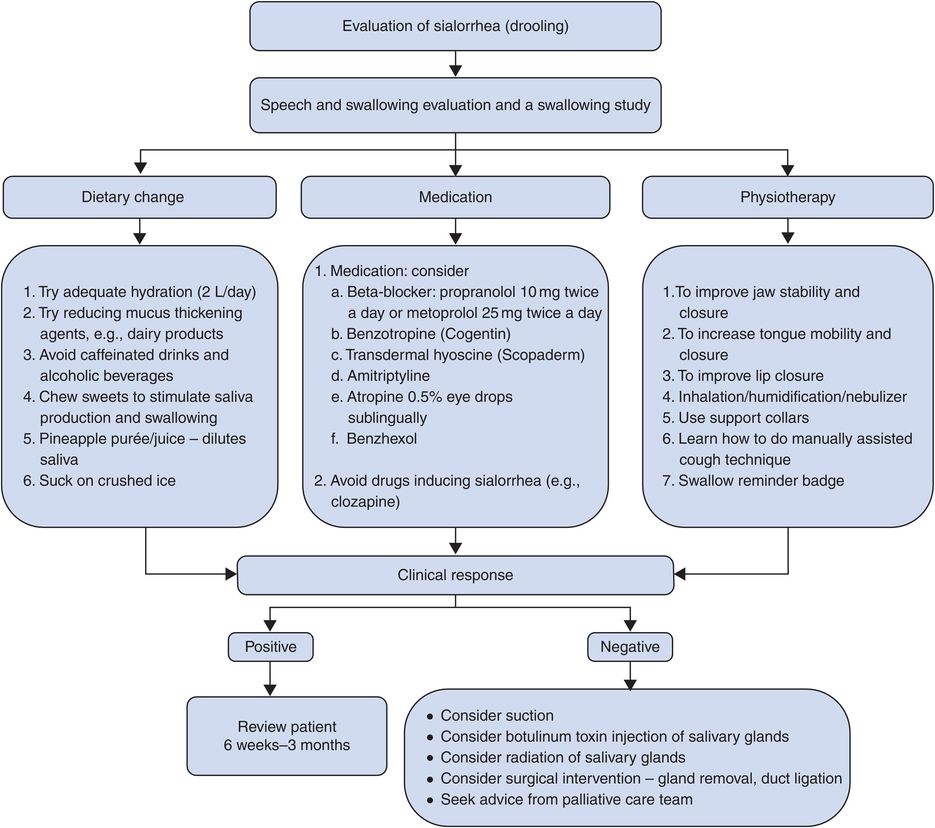
Sialorrhea
Increasing sialorrhea as an adverse effect of STN DBS has been reported.152,238 This issue may also be complicated by the coexistence of worsening in cognitive function, which may contribute to a reduced control of secretions. Increased drooling has been reported in patients who underwent STN DBS.239 It is unclear whether this is an effect of DBS or if there are other factors, such as natural progression of disease or modification of medication regimen, that may influence outcome.
Troubleshooting
Most practitioners use anticholinergics and/or botulinum toxin in a case-by-case manner to treat this issue. Some practitioners use atropine drops under the tongue (Figure 12.15).
Dysphagia
Dysphagia has been reported in 4–8% of patients after STN DBS.148,156,158 It remains unclear whether this results directly from the DBS or if this change reflects disease progression and involvement of non-dopaminergic pathways. Troche et al. have performed a systematic review of all experimental studies of swallowing post-DBS and identified nine pertinent studies.240 All of these studies were only performed in those with STN DBS, and all were either bilateral or pooled bilateral and unilateral cases. There was no convincing evidence that swallowing was affected, but some measures of pharyngeal swallowing were noted to be worse. In a more recent series, a retrospective analysis of 33 patients who had unilateral DBS in either STN or GPi suggested that those having STN DBS performed worse on swallowing outcomes, although this study had limitations.241 Taken together, there exists a paucity of studies addressing this question, and follow-up will require carefully designed studies to control for target, approach (unilateral versus bilateral), and outcome measures. In the experience of many clinicians, there are patients who report changes in swallowing after DBS. In many cases, however, the spread of DBS current to corticospinal and corticobulbar fibers beyond the intended target may be responsible for worsening of swallowing.
Troubleshooting
A swallowing study needs to be performed in both “on” and “off” stimulation conditions if DBS-related swallowing problems are suspected. Reprogramming DBS parameters and performing medication adjustments may benefit some patients. Swallowing therapy and recommendations from a specialist can prevent aspiration. A referral to a nutritionist may be helpful for dietary evaluation and adjustment.
Hypersexuality
The prevalence of hypersexuality in PD is estimated at 2.4%.159 Most studies160,242–244report that men treated with STN DBS have an increased frequency, increased satisfaction, and decreased avoidance of sexual activity, especially those younger than 60 years of age. There were no significant changes observed in sexual functioning for women post-DBS, although this population has been less studied. The mechanism for excessive sexual arousal after DBS is currently unknown.
Troubleshooting
Reducing dopaminergic medications245 and/or reprogramming DBS parameters may be beneficial. It is important to determine whether the hypersexuality is a manifestation of an ICD. Atypical antipsychotic medication246 can be used in select cases. Donepezil hydrochloride also may aid in some cases of compulsive hypersexuality.247
Weight gain
Weight gain is common after DBS at the STN or GPi targets. It occurs at an incidence of 6–100% after STN DBS130,148,160and 26–96% after GPi DBS.58 There have been no reports regarding weight gain after Vim DBS, but this phenomenon has been less studied. Most patients will have a weight gain of approximately 9.3–9.7 kg and an increase in body mass index by 4.7 kg/m2 one year post STN DBS treatment.248,249
There are several explanations for weight gain following STN DBS including: (1) changes in dopaminergic medications that may increase the drive for food intake or contribute to an ICD underlying the issue and leading to binge eating; (2) decreased energy expenditure may occur due to less motor fluctuation and dyskinesia; (3) normalization of energy metabolism following STN implantation may favor body weight gain (men gained mass, while women gained fat);250 (4) spread of electrical current into nonmotor regions, such as the hypothalamus, can induce changes in eating behavior; (5) other factors, such as changes in living style and physical activities, may be involved.
Troubleshooting
Nutritional counseling to prevent rapid and excessive weight gain, as well as the initiation of an exercise program, can be useful.
Problems specific to GPi DBS (Figures 12.16 and 12.17)
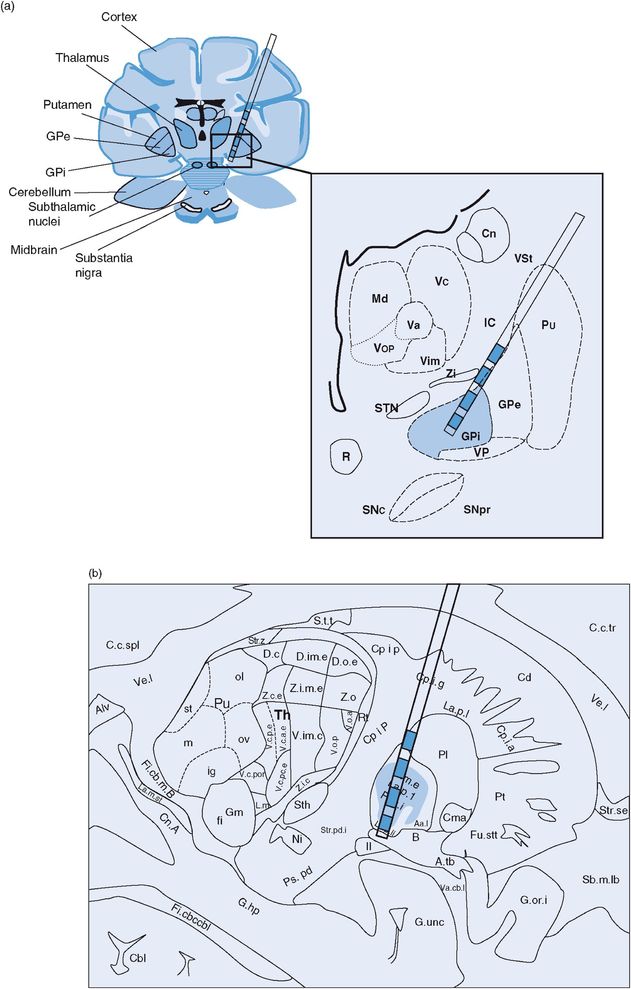
GPi DBS lead location in (a) coronal and (b) sagittal planes.
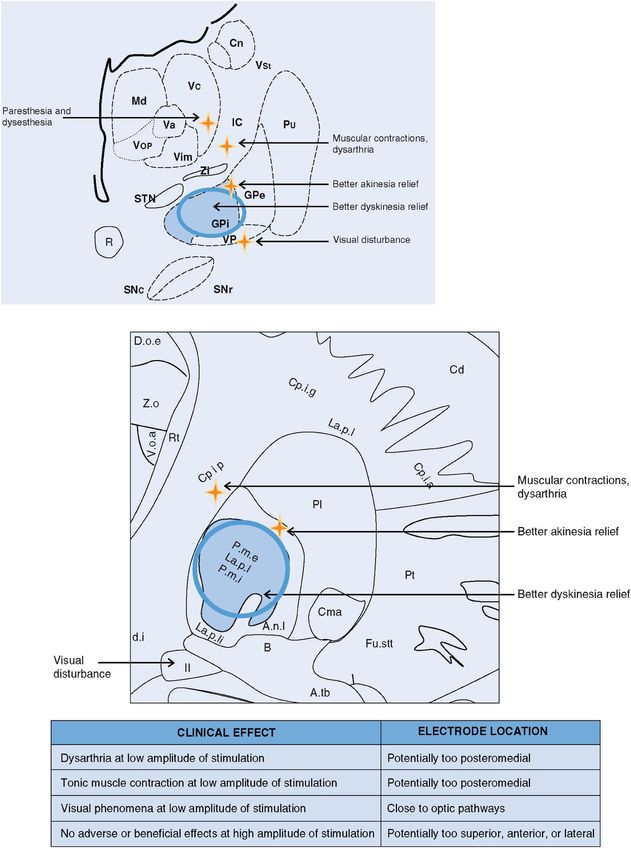
GPi DBS: stimulation-induced effects. Cn, caudate nucleus; GPe, globus pallidus externus; GPi, globus pallidus internus; IC, internal capsule; Md, nucleus mediodorsal thalamus; Pu, putamen; R, red nucleus; SNc, substantia nigra pars compacta; SNpr, substantia nigra pars reticulata; STN, subthalamic nucleus; Va, nucleus ventral anterior thalamus; Vc, nucleus ventrocaudal thalamus; Vim, nucleus ventral intermediate thalamus; VP, visual pathway/optic tract; Vop, nucleus ventral oral posterior thalamus; VSt, ventral striatum; Zi, zona incerta.
Muscle contraction
Muscle contraction with GPi DBS generally results from current spread into the internal capsule.207 The internal capsule is located posteriorly and medially to the GPi. Clinicians need to be very careful to distinguish between tonic muscle contraction and dystonia related to the underlying movement disorder, which can be challenging.
Troubleshooting
During DBS programming, the clinician needs to attempt to differentiate dystonia from capsular effects (that is, an effect caused by spread of the stimulation into the fibers of the internal capsule). If a capsular effect is elicited, reprogramming at lower amplitude or pulse width or changing the electrode configuration may be performed. If low-threshold capsular responses prevent adequate stimulation, then DBS lead revision may be considered.
Dysarthria/hypophonia
Dysarthria/hypophonia with GPi DBS may possibly occur less frequently than in STN DBS (particularly if unilateral GPi is used), although it has been reported.131,161 Speech and swallowing examinations conducted both on and off stimulation may aid in the diagnosis.
Visual phenomena
During intraoperative microelectrode recording, identification of the optic tract is used to help determine the ventral border of GPi.251 If the DBS lead is implanted near the optic tract and electrical current spreads to the optic tract region, the patient may report seeing sparkles of light (phosphenes). Homonymous quadrantanopsia can occur after GPi DBS from a lesion of the optic tract, but this is rare in DBS (unless a stroke or hemorrhage has occurred) and is more commonly encountered in patients undergoing the ablative procedure of pallidotomy.252
Troubleshooting
The persistence of phosphenes can usually be managed by using a more dorsal electrode for stimulation, by reprogramming, or by repositioning the DBS lead if stimulation parameter adjustment fails to resolve this problem.144
Status dystonicus
There have been isolated case reports of status dystonicus, or dystonic storm, associated with sudden DBS failure or associated with changes in stimulation parameters.162 This phenomenon has been reported with pallidal stimulation, but presumably sudden cessation of STN DBS could also produce this issue.
Troubleshooting
In cases of unexpected failures or abrupt changes in stimulation, providing a set of stimulation parameters that can be gradually ramped up, or providing an adjustable amplitude range may facilitate the patient returning to their original settings over time. Abrupt reactivation to original DBS settings can also provoke dystonic storm (Okun, personal communication).
Troubleshooting other DBS-related issues
Disease progression
Worsening of symptoms may occur over time, even when the patient has been deriving benefit from DBS for years. This may simply signify progression of the disease and may not necessarily be indicative of device failure. In essential tremor, there may be loss of benefit with long-term DBS, often attributed to tolerance; however, this is more likely to reflect disease progression and efforts should be made to rule out suboptimal lead placement.253
Troubleshooting
Imaging studies should be performed to determine if there has been migration of the DBS lead (an uncommon occurrence). Re-examining the patient using standardized rating scales can also be useful. For patients with PD, assessment of the UPDRS examination while “off” medication and “off” stimulation and then again while “on” medication and “off” stimulation will potentially identify levodopa-responsive symptoms. If the symptom does not improve with levodopa, then it is also unlikely to respond to changes in DBS programming or lead placement (the notable exceptions being tremor and dyskinesia).49,116
Poor DBS candidate/lack of evaluation by an interdisciplinary team
Ideally, an interdisciplinary team should be involved in selecting patients for treatment with DBS.116,254,255
This team typically consists of a movement disorder-trained neurologist and neurosurgeon, a nurse and/or nurse practitioner, a physician assistant, a psychiatrist, a neuropsychologist, an occupational therapist, a nutritionist, a speech pathologist, a social worker, a financial counselor, and a home care case manager. Each team member independently assesses the patient, and the team then meets to review and discuss findings in a team setting. In the meeting, the interdisciplinary team will determine whether patients are suitable for treatment with DBS, develop a risk–benefit profile, and discuss the safest operative approaches (staged versus simultaneous lead insertion, DBS target, etc.). As discussed in detail in Chapter 2, a variety of criteria are considered in determining patient candidacy for treatment with DBS. For those with PD, patients need to have a clear diagnosis of idiopathic PD (preferably diagnosed by a movement disorder specialist256). They should not have atypical parkinsonian features. Patients should demonstrate a clear (even if only brief) response to dopaminergic medication, with improvement in the UPDRS motor score of at least 30% between the off- and on-medication states.257 There should be absence of active psychiatric illness and little or no cognitive impairment. Other considerations, such as age, general health, and patient expectations, should also be reviewed (Table 12.5).
| Diagnosis | Ideal characteristics for DBS | Potentially unfavorable characteristics for DBS |
|---|---|---|
| Parkinson’s disease |
|
|
| Dystonia |
|
|
| Essential tremor |
|
|
Notes: DBS, deep brain stimulation; PD, Parkinson’s disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree