Fig. 10.1
Example of a typical sleep diary
Symptom inventories provide clinicians with dimensional measures of frequency and severity, which provide necessary information to inform accurate diagnosis and assessment of response to clinical intervention. Insomnia symptom severity can be quantified by using the Insomnia Severity Index (ISI) [23], a seven-item self-report inventory that reliably identifies clinically significant symptoms of insomnia (i.e., cutoff scores of 10, 11, and 14 have been identified for community, clinical, and primary care populations, respectively) [31, 32], and it can be used to monitor clinically significant change over the course of treatment (i.e., seven-point reduction indicates the minimally important difference to be considered moderately improved; scores ≤7 indicate remission) [32]. The addition of qualitative measures of sleep-wake functioning like the Epworth Sleepiness Scale (ESS) [33] and the PROMIS Sleep Disturbance and Sleep-Related Impairment short forms [34] may be used to quantify functional impairment and may potentially identify comorbid sleep disorders that cause daytime sleepiness. Inclusion of brief self-report instruments to screen for depression (e.g., PHQ-9 [35] or Quick Inventory for Depressive Symptomatology [36]), anxiety (Generalized Anxiety Disorder scale [GAD-7] [37] or the trait subscale of the State Trait Anxiety Inventory [38]), and PTSD (Life Events Checklist [39] and the PTSD Checklist [40]) is warranted given the high rate of co-occurrence of these disorders with insomnia.
Polysomnographic (PSG) testing and actigraphy monitoring are not considered routine assessments for an insomnia evaluation. PSG in particular generally does not provide relevant information for confirming or excluding the presence of insomnia [41]. However, for patients with a primary complaint of nonrestorative sleep or sleep maintenance insomnia with collateral information from a bed partner regarding observations of sleep-disordered breathing, periodic limb movements during sleep, or excessive daytime somnolence, consideration of a PSG is warranted. Actigraphy may be helpful in ruling out a circadian rhythm sleep disorder, monitoring adherence to treatment recommendations, and assessing objective sleep duration for extended periods of time in the home environment [42]. Both PSG and actigraphy may be useful in cases of sleep state misperception (also known as paradoxical insomnia), a condition characterized by objectively normal sleep duration, continuity, and architecture despite patient complaints of gross sleep disturbances and minimal daytime impairment. It is noteworthy that individuals with insomnia generally underestimate sleep duration and overestimate sleep onset latency and awakenings relative to good sleepers. In addition, individuals with insomnia frequently have normal daytime alertness and longer sleep latencies on the multiple sleep latency test [43, 44]. Taken together these data support the role of physiological hyperarousal in insomnia.
Diagnosis
Diagnostic classification systems are necessary for defining criteria used in the diagnosis and treatment of insomnia, facilitate communication between providers, and provide a basis for billing. Variability between sleep-wake nosologies reflects the idiosyncratic nature of classification systems in general and a need to standardize a uniform diagnostic classification system. Contemporary nosologies describing sleep-wake disorders (e.g., Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [45], International Classification of Diseases (ICD-10) [46], and International Classification of Sleep Disorders, 3rd edition (ICSD-3) [47]) characterize insomnia as global sleep dissatisfaction associated with difficulty initiating or maintaining sleep despite adequate opportunity (e.g., enough time allotted for sleep) and circumstances (e.g., suitable sleep environment) for sleep with clinically significant daytime impairment (e.g., daytime fatigue, psychological distress, cognitive impairment, concerns/worry about sleep). In children, these sleep difficulties are often reported by a caregiver and may include observations of bedtime resistance or difficulty sleeping independently (i.e., sleep onset association) without caregiver intervention [48]. Inclusion of an adequate opportunity and circumstances for sleep differentiates insomnia from sleep deprivation. Epidemiological and longitudinal studies support the DSM-5 and ICSD-3 specification of sleep difficulties occurring with a frequency of at least three nights a week [5, 49] with additional temporal specifiers included for duration of symptoms including episodic/short-term insomnia (symptoms lasting ≥1 month but <3 months) and persistent/chronic insomnia (symptoms lasting ≥3 months) [50]. Individuals with a history of recurrent episodes over several years would be subsumed under the persistent/chronic diagnosis given the duration of symptoms. It is noteworthy that earlier classification systems included nonrestorative sleep (e.g., sleep that is of poor quality or unrefreshing) as an independent insomnia criterion, but these complaints alone do not suffice as the sole sleep complaint in current nosologies.
Historically within clinical practice insomnia subtypes have been characterized by the nature of their sleep complaints as early, middle, and late based on perceived difficulty with sleep initiation, sleep maintenance difficulty during the night, and sleep maintenance difficulty at the end of the night, respectively. Additional categorization schemes of primary versus secondary age of onset (i.e., childhood vs. adult) have served as useful heuristics, but they are categorizations of convenience lacking empirical support or biological basis and have yielded low diagnostic reliability [51, 52]. Classification of insomnia based on objective sleep duration has yielded perhaps a viable means of distinguishing insomnia subtypes. Insomnia with objective short sleep duration (i.e., <5–6 h of objective sleep) is associated with cognitive-emotional, cortical, and physiological hyperarousal and health problems including diabetes, hypertension, increased mortality, and neuropsychological deficits [53] relative to insomnia with longer sleep duration (i.e., >6 h of objective sleep). It is noteworthy from a clinical perspective that individuals reporting short sleep durations of 4 h or less are at high risk for early termination of group or individual therapy [54].
Differential Diagnosis
An insomnia diagnosis needs to be differentiated from normal sleep variants, circadian rhythm sleep disorders, insufficient sleep, and other sleep disorders. As there is a continuum of sleep need, short sleepers can be differentiated from insomnia by the absence of sleep initiation and maintenance complaints and daytime impairments. Two circadian rhythm sleep disorders, delayed sleep phase type and advanced sleep phase type, are characterized by sleep difficulties arising from misalignment between preferred sleep schedules and the endogenous circadian rhythm. Delayed sleep phase type is differentiated from insomnia because individuals with insomnia feel sleepy at their desired bedtime but are unable to sleep regardless of their bed and rise times whereas an individual with delayed sleep phase type will report normal sleep duration with little to no sleep initiation or maintenance complaints when allowed to sleep on their internally preferred schedule. Delayed sleep phase type is more common among adolescents and young adults. Individuals with insomnia report sleep maintenance problems and early morning rise times regardless of sleep schedule whereas sleep duration and continuity is normal for an individual with advanced sleep phase type when sleeping in phase (i.e., early bed and wake time). Advanced sleep phase type is more common in older adults than children or young adults. A differential diagnosis may be enhanced by having patients complete self-reported circadian rhythm characteristics such as the Horne and Östberg Morningness-Eveningness Questionnaire (MEQ) [55] or the Munich ChronoType Questionnaire (MCTQ) [56]. Insufficient sleep syndrome is differentiated from insomnias by the presence of excessive daytime somnolence, which is not typical in chronic insomnia. In addition, the chronic sleep restriction associated with insufficient sleep syndrome is volitional whereas those with chronic insomnia have reduced total sleep times due to sleep initiation and maintenance problems in the context of adequate opportunity and circumstances for sleep. Finally, as discussed above, other sleep disorders including sleep-disordered breathing and periodic limb movement disorder may manifest as sleep maintenance problems, and restless legs syndrome may contribute to sleep initiation or maintenance complaints.
Treatment
Pharmacological Interventions
Nationally representative samples of adults in the USA indicate use of prescription medication to treat insomnia has increased over time [15, 57] with approximately 3 % of adults reporting the use of medication to treat insomnia symptoms [15] and 25 % of these individuals treating their insomnia for 4 months or longer [6]. Hypnotics remain first-line treatments for chronic insomnia in primary care because they are widely available, are easy to prescribe, provide immediate symptomatic relief, and are efficacious in the short term [58]. When addressed in primary care settings, insomnia is typically treated with FDA-approved hypnotics including benzodiazepine receptor agonists, nonbenzodiazepine receptor agonists, melatonin receptor agonists, antihistamines, and antidepressants [59–64] (Table 10.1). Benzodiazepine and nonbenzodiazepine receptor agonists generally have similar efficacy on PSG and sleep diary outcomes [65]. Several medications with sedating properties used to treat anxiety, depression, and seizures are commonly used off-label to treat insomnia, however, efficacy data is limited [59]. Patients often seek over-the-counter medications containing antihistamines or natural products (e.g., melatonin, l-tryptophan, valerian), but the FDA does not regulate herbs and supplements; therefore, patients may be unaware that the dose and purity of a given product is not monitored. Over-the-counter medications and supplements are generally not recommended due to limited efficacy and safety data [27]. Available data on antihistamines suggests that tolerance to these medications likely develops quickly [66]; therefore, use should be short term. The limited evidence for evaluating exogenous melatonin for insomnia indicates relatively modest benefit as reflected in relatively small effect sizes for reductions in sleep onset latency and increased sleep efficiency and total sleep time relative to benzodiazepine and nonbenzodiazepine receptor agonists [60]. Nonbenzodiazepine receptor agonists (1.23 % of the US population) are the most commonly prescribed medications for insomnia followed by trazodone (0.97 %), benzodiazepines (0.40 %), quetiapine (0.32 %), and doxepin (0.12 %). It is not uncommon to be prescribed additional sedative medications, with one study indicating that up to 55 % of patients take an additional sedative hypnotic (e.g., opioid or benzodiazepine) and 10 % take three or more [15]. It is noteworthy that there is no medication prescribed in the USA with FDA approval for use in pediatric patients for the treatment of insomnia, but pediatric patients frequently are prescribed medications off-label to treat insomnia, with antihistamines (33 %), benzodiazepine (26 %), alpha-2 agonists (e.g., clonidine, guanfacine; 15 %), and antidepressants (6 %) commonly recommended [67]. The timing of medication is also critical for optimal treatment, with minimal side effects occurring when taken within close proximity to bedtime. However, estimates indicate that up to 20 % of individuals use their hypnotic medication in the middle of the night [68]. This practice can be quite problematic given that an adequate opportunity to sleep is recommended when using hypnotics given their long half-life (e.g., 1.5–100 h for benzodiazepines and 1–6 h for nonbenzodiazepine receptor agonists). Individuals utilizing medication in the middle of the night are vulnerable to next-day hangover effects, including cognitive impairment and psychomotor impairment. Furthermore, benzodiazepines carry the risk of tolerance, dependence, and withdrawal effects such as rebound insomnia [69]. Relative to placebo, nonbenzodiazepine receptor agonists and sedating antidepressants are associated with higher rates of headache, somnolence, dizziness, and nausea [65]. Both benzodiazepine and nonbenzodiazepine receptor agonists are associated with complex sleep-related behaviors such as sleep walking, aggressive behavior, eating, and driving. Therefore, care is required in coordinating pharmacological interventions to minimize potentially sedating side effects, which may increase risk of injury.
Table 10.1
Common pharmacological interventions for insomnia
Medication | Brand name | Peak concentration (h) | Half-life (h) | Recommended dose for adults (elderly) | Indication |
|---|---|---|---|---|---|
Benzodiazepine | |||||
Temazepama | Restoril | 1.2–1.6 | 8–10 | 7.5–30 mg (7.5 mg) | Sleep onset and maintenance |
Triazolama | Halcion | 2 | 1.5–5.5 | 0.25–0.5 mg (0.125–0.25 mg) | Sleep onset and maintenance |
Estazolama | Prosom | 0.5–6 | 10–24 | 1–2 mg (0.5–1 mg) | Sleep onset and maintenance |
Benzodiazepine receptor agonist (BZRA) | |||||
Eszopiclonea | Lunesta | 1 | 6 | 2–3 mg (sleep initiation = 1 mg; sleep maintenance = 2 mg) | Sleep onset and maintenance |
Zaleplona | Sonata | 1 | 1 | 10 mg (5 mg) | Sleep onset |
Zolpidema | Ambien | 1.6 | 2.5 | Men, 5–10 mg; women, 5 mg (5 mg) | Sleep onset |
Zolpidem (extended release)a | Ambien CR | 1.5 | 2.5 | Men, 6.25–12.5 mg; women, 6.25 mg (6.25 mg) | Sleep onset and maintenance |
Zolpidem (sublingual)a | Intermezzo | 0.5–1.25 | 1.4–3.6 | Men, 3.5 mg; women, 1.5 mg (1.75 mg) | Sleep maintenance |
Melatonin receptor agonist | |||||
Ramelteona | Rozerem | 0.5–1.5 | 1–5 | 8 mg | Sleep onset |
Antidepressant | |||||
Doxepina | Sinequan | 3.5 | 15 | 6 mg (3 mg) | Sleep maintenance |
Trazodone | Desyrel | 1.5 | 7 | 25–75 mg | Sleep onset and maintenance |
Anticonvulsant | |||||
Gabapentin | Neurontin | 2.5 | 5–7 | 300–600 (300) | Sleep maintenance |
Antipsychotic | |||||
Quetiapine | Seroquel | 2 | 6 | 25–200 (25) | Insufficient data [59] |
Over-the-counter sleep aids | |||||
Diphenhydraminea | Benadryl | 2–2.5 | 5–11 | 50–75 mg (25 mg) | Sleep onset and maintenance |
Melatonin | 0.3–1 | 0.5 | No recommendation; typical range reported by patients: 0.3–10 mg | Sleep onset | |
In the short term hypnotics serve as an appropriate measure for treating insomnia complaints. However, insomnia is often chronic, making short-term use of hypnotics a less favorable treatment option. In addition, there is limited evidence to support the long-term use of hypnotics with many placebo-controlled studies lasting for only 4 weeks or less in non-elderly adults [65, 70] and very few studies lasting 12 months [71–73]. Hypnotics may be contraindicated in individuals with medical histories including alcohol or sedative abuse/dependence and untreated sleep apnea [74]. Many patients report a strong desire to discontinue use of hypnotic medications [75], and this should be done gradually (e.g., 25 % reduction/week) under the supervision of a physician to minimize withdrawal effects (e.g., rebound insomnia).
Non-pharmacological Interventions
Non-pharmacological interventions for insomnia are intended to promote greater control over sleep, reduce emotional distress, reduce variability in sleep schedules, consolidate sleep, and enhance sleep efficiency. These interventions have well-established short-term and long-term efficacy [76, 77] and seem to have fewer side effects [78] than pharmacological interventions for insomnia. Behavioral strategies including sleep restriction therapy and stimulus control therapy (Table 10.2) meet established “standard” treatment criteria as defined by the American Psychological Association criteria for empirically validated treatments [79] or consensus recommendations made by the American Academy of Sleep Medicine as effective and evidenced-based interventions for insomnia [80]. These strategies are typically combined and supplemented with cognitive therapy and sleep education as a multicomponent cognitive behavioral therapy for insomnia (CBT-I), which is often considered more acceptable than pharmacological treatment by patients [81]. The efficacy of non-pharmacological interventions has been reviewed in several meta-analyses [76, 77, 82–85] which have consistently indicated that 50–70 % of treated patients benefit from intervention with medium to large effect sizes observed for sleep onset latency, wake time after sleep onset, sleep efficiency, and sleep quality ratings (Fig. 10.2). Effect sizes for total sleep time at the end of intervention are small but improve during follow-up periods with medium effect sizes [82, 84, 85]. Initial treatment gains maintained during follow-up periods for as long as 2–3 years [78, 86, 87]. Although CBT-I yields effective changes in sleep, categorical response and remission treatment outcomes are difficult to determine because no universally accepted criteria have been established [30]. Several trials have utilized the ISI [88–90], sleep efficiency >85 % [87], or a combination of sleep efficiency and sleep quality ratings [91], with overall report of response rates of 52–65 % and remission rates of 32–55 % [89–91]. Indeed the establishment of guidelines will guide clinical practice and future effectiveness trials. The evaluation of treatment outcomes has also unfortunately focused primarily on sleep-related variables rather than daytime functioning, a cardinal feature of insomnia and a principal factor influencing those seeking treatment. It is critical to evaluate daytime functioning to further assess response and remission, and the use of an instrument like the PROMIS Sleep-Related Impairment questionnaire may help evaluate response and remission multidimensionally.
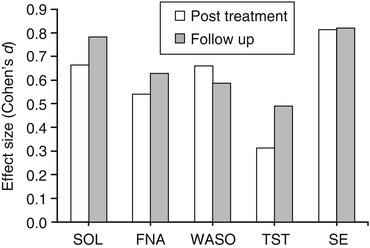
Table 10.2
Non-pharmacological interventions for insomnia
Technique | Recommended standard of care | Description |
|---|---|---|
Stimulus control | Standard | Standardized instructions intended to reassociate the bed/sleep environment with sleep rather than sleep-incompatible behaviors (e.g., boredom, frustration, or worry at not being able to sleep) and to reestablish a consistent sleep/wake schedule |
Sleep restriction | Standard | To increase sleep drive and consolidate sleep, patient’s time in bed is limited to the total sleep time derived from 1 to 2 weeks of sleep diary. Time in bed is subsequently titrated up or down based on response to intervention, and minimum time in bed is generally never less than 5 h and is applied flexibly based on acceptance and adherence to treatment. Prohibits naps at times other than the assigned time in bed |
Relaxation training | Standard | Strategies aimed at reducing somatic tension or intrusive thoughts at bedtime interfering with sleep. Common techniques include diaphragmatic breathing, progressive muscle relaxation, meditation, mindfulness, and imagery |
Cognitive behavioral therapy for insomnia (CBT-I) | Standard | A combination of behavioral (e.g., stimulus control, sleep restriction, relaxation) and cognitive interventions |
Paradoxical intention | Guideline | A cognitive treatment approach targeting the preoccupation with the effort and ability to sleep that is believed to precipitate an aroused state incompatible with sleep. Patients are instructed to remain passively awake without any effort to fall asleep rather than trying to fall asleep. This strategy is repeated as necessary if patient awakens during the night |
Biofeedback | Guideline | A form of relaxation coupled with sensory feedback to help patients learn to control a physiological parameter (e.g., muscle tension, EEG) to reduce somatic arousal |
Cognitive therapy | No recommendation | Strategies aimed at challenging and changing dysfunctional attitudes and beliefs, misconceptions about sleep, and beliefs about insomnia and its perceived daytime consequences that contribute to emotional distress and further sleep problems |
Sleep hygiene therapy | No recommendation | Guidelines about healthy sleep practices (e.g., diet, exercise, substance use) and environmental factors (e.g., light, noise, temperature) that may promote or interfere with sleep. Typically includes psychoeducation about normative sleep and changes in sleep patterns associated with aging |

Fig. 10.2
Mean effect size estimates (Cohen’s d) of sleep diary parameters derived from meta-analyses [76, 82–85] with d = 0.20, d = 0.50, and d = 0.80 indicating small, medium, and large effects, respectively. SOL indicates sleep onset latency, FNA indicates frequency of nocturnal awakenings, WASO indicates wake after sleep onset time, TST indicates total sleep time, and SE indicates sleep efficiency
The efficacy and safety of CBT-I have been documented not only in numerous controlled trials of patients with primary insomnia but also in patients with insomnia and co-occurring conditions commonly seen in primary care, such as depression and other psychiatric disorders [92, 93], chronic pain [94–96], breast cancer [97, 98], and hypnotic dependence [75]. Head-to-head comparisons of CBT-I and pharmacotherapy found that ratings of sleep quality were equivalent or better for CBT-I treated patients at posttreatment and superior 1 and 6 months following treatment [78, 99]. Although smaller in magnitude, improvements in objective sleep quality measured by actigraphy [91] and polysomnography [87, 90] have also been consistently found with CBT-I. CBT-I offers several potential advantages over pharmacotherapy, including greater patient preference [81, 100], equal to superior outcomes after 6–8 weeks of therapy [78, 99, 101], more evidence of sustained treatment efficacy (e.g., [86, 87]), fewer adverse side effects and contraindications, and no concerns about drug interactions. However, sleep restriction therapy, a common component of CBT-I, may increase somnolence and decrease psychomotor vigilance [102]. Therefore, it is recommended that providers advise patients to exercise caution when engaging in sleep-sensitive tasks (e.g., driving, operating machinery). Moreover, providers are also encouraged to consider the limitations of CBT-I’s primary behavioral components, sleep restriction, and stimulus control for individuals with conditions that may be exacerbated by sleep resection, including bipolar disorder, epilepsy, parasomnias, and psychotic disorders. Also stimulus control may not be appropriate for individuals who are disabled, cannot get out of bed easily unassisted, or are at risk of accidental injury.
Despite several strengths, dissemination of CBT-I is limited by the dearth of clinicians trained to deliver these interventions [103], access to sleep disorders centers, and cost. Relative to pharmacotherapy, CBT for insomnia is perceived to be more burdensome, with a typical course requiring 5–6 face-to-face sessions over a 6–7-week period [77]. Another significant barrier is that providers trained to deliver CBT-I are typically not located in primary care. Instead, patients who prefer CBT-I over hypnotics must find one of the few qualified providers in the country (fewer than 200 providers are certified in Behavioral Sleep Medicine nationwide [http://www.absm.org/BSMSpecialists.aspx], a designation that denotes expertise in CBT for insomnia), most of whom are located in departments of psychology/psychiatry or affiliated with sleep disorders centers in major urban areas. Finally, CBT-I is often criticized as being more costly than pharmacotherapy. Using 2002 reimbursement rates, one study estimated that 8 weeks of group (4–6 patients) CBT-I costs $279.92 compared with $255.30 for 100 tablets of zolpidem 10 mg. These estimates predate the availability of zolpidem in generic form, meaning that short-term medication costs are substantially lower. On the other hand, they also fail to consider that CBT-I is time limited whereas hypnotic use frequently exceeds 8 weeks [104]. No study has conducted prospective comparative cost-effectiveness analyses of available insomnia therapies despite the importance of such information [105]. Addressing these major barriers—treatment modality, provider, and cost—is likely critical for CBT-I to be adopted as a first-line insomnia therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





