(1)
Cognitive Function Clinic, Walton Centre for Neurology and Neurosurgery, Liverpool, UK
5.2.3 Addenbrooke’s Cognitive Examination (ACE) and Addenbrooke’s Cognitive Examination-Revised (ACE-R)
5.4.2 AD8
Abstract
This chapter examines the diagnostic utility of various non-cognitive screening instruments, examining functional, behavioural and psychiatric, neurovegetative, and informant scales, in the diagnosis of cognitive disorders. Methods to compare these tests in terms of various diagnostic parameters are also considered. The diagnostic utility of combinations of scales is also examined.
Keywords
DementiaDiagnosisNon-cognitive screening instrumentsCombinationsThe dementia syndrome may comprise more than simply cognitive decline (American Psychiatric Association 2000), hence there may be a necessity to examine functional, behavioural, and neurovegetative domains as well as cognition in patients suspected to have a dementing disorder. This is consistent with a biopsychosocial model of disease (Engel 1977), and the exploration of these domains contradicts assertions that neurologists subscribe to a purely medical model of dementia. Furthermore, dementia has important differential diagnoses with affective disorders (especially anxiety and depression; see Sect. 5.2) and with delirium (Larner 2004). These differentials are not necessarily straightforward since the conditions may coexist, for example delirium is sometimes the presenting feature of an underlying neurodegenerative disorder (e.g. Rockwood et al. 1999), and depression is sometimes a precursor of dementia.
The same methodology as used for assessment of the utility of neurological signs and cognitive screening instruments (see Chaps. 2, 3 and 4) may be applied to scales examining non-cognitive domains. All the studies reported here predate the publication of DSM-5 in 2013 and hence DSM-IV diagnostic criteria are used where applicable throughout.
5.1 Functional Scales
The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) defined dementia as “the development of multiple cognitive deficits that include memory impairment (criterion A1)… sufficiently severe to cause impairment in occupational or social functioning (criterion B)” (American Psychiatric Association 2000:148,149,157). Hence, impairments in activities of daily living (ADLs) would appear, according to this definition, to be a sine qua non for the diagnosis of dementia. However, instruments used to assess social and occupational functions (the specific examples given in DSM-IV are going to school, working, shopping, dressing, bathing, handling finances) have seldom been used for diagnostic purposes, although they may often be used to plan appropriate care interventions for people with dementia. Pfeffer et al. (1982) used the Functional Activities Questionnaire (FAQ) for the diagnosis of dementia in a “stable retirement community” in California, finding a sensitivity of 0.85 and specificity of 0.81 (hence, retrospectively calculated LR+ = 4.47; LR− = 0.19; Hancock and Larner 2007). They also administered Lawton and Brody’s (1969) Instrumental Activities of Daily Living (IADL) Scale and reported a sensitivity of 0.57 and specificity of 0.92 (retrospectively calculated LR+ = 7.13; LR− = 0.46; no cutoff explicitly stated in the text; Hancock and Larner 2007). The paucity of studies examining ADL scales for diagnosis of dementia may be related to shortcomings in the extant scales (Sikkes et al. 2009); newer scales such as the Amsterdam IADL Questionnaire may obviate some of these problems (Sikkes et al. 2013).
5.1.1 Instrumental Activities of Daily Living (IADL) Scale
The Instrumental Activities of Daily Living (IADL) Scale assesses six basic ADLs (also known as the Physical Self-Maintenance Scale) and eight instrumental ADLs (Box 5.1) in a hierarchical manner according to degree of autonomy (Lawton and Brody 1969). It is reported to have good reliability and validity (Hokoishi et al. 2001).
Box 5.1: Item Content of Instrumental Activities of Daily Living (IADL) Scale
Instrumental ADL:
Ability to use telephone
Shopping
Food preparation
Housekeeping
Laundry
Mode of transportation
Responsibility for own medications
Ability to handle finances
Basic ADL (Physical Self-Maintenance Scale):
Toileting
Feeding
Dressing
Grooming
Physical ambulation
Bathing
The diagnostic utility of the IADL Scale in the diagnosis of dementia in day-to-day clinical practice has been assessed prospectively in new referrals to CFC and to the Brooker Centre, Runcorn, over a 2-year period (February 2004-February 2006) (Hancock and Larner 2007; Larner and Hancock 2008a). Scoring of each ADL domain was by forced choice, either 0 (dependent) or 1 (independent), giving a score range of 0–14.
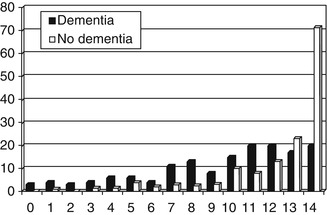
A total of 296 patients were assessed of whom 154 were judged to be demented. The most common cause of dementia was Alzheimer’s disease (AD) or mixed AD/cerebrovascular disease (n = 122; 79 %), with smaller numbers due to vascular dementia (13), frontotemporal lobar degeneration syndromes (FTLD; 11), and miscellaneous other causes (8). The IADL Scale proved easy to use, being completed in all cases, usually in under 5 min, often with the assistance of an informant (n = 233), including spouse (130), other relative (84) – most often a child (60/84) – carer (10) or friend (9). The distribution of IADL scores is shown in Fig. 5.1.
Diagnostic utility proved suboptimal (Table 5.1) with only sensitivity achieving the desired value of >80 % (The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group 1998). The relative risk or risk ratio for impaired ADL, defined by IADL scale cutoff score of ≤13/14, in non-demented compared to demented individuals was 0.57 (95 % CI = 0.48–0.68).
IADL | |
|---|---|
N | 296 |
Prevalence dementia | 52 % |
M:F (% male) | 145:151 (49) |
Age range in years | 23–90 (median 64) |
Cutoff | 13/14 |
Accuracy | 0.69 (0.64–0.75) |
Sensitivity (Se) | 0.87 (0.82–0.92) |
Specificity (Sp) | 0.50 (0.42–0.58) |
Y | 0.37 |
PPV | 0.65 (0.59–0.72) |
NPV | 0.78 (0.70–0.87) |
PSI | 0.43 |
LR+ | 1.74 (1.46–2.07) = unimportant |
LR− | 0.26 (0.22–0.30) = small |
DOR | 6.70 (5.62–7.98) |
CUI+ | 0.57 (adequate) |
CUI− | 0.39 (poor) |
AUC ROC curve | 0.75 (0.72–0.78) |
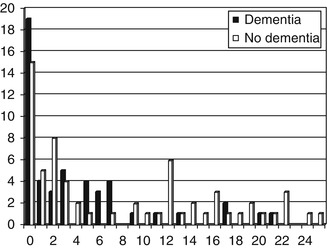
Subscores of the IADL Scale were also examined, namely the instrumental activities part only (score range 0–8), and the 4-IADL scale as defined by Barberger-Gateau et al. (1992), namely ability to use telephone, use public/private transport, handle own medications, and handle finances (score range 0–4). Neither of these subscores produced better results in terms of diagnostic utility (see Fig. 5.2 for 4-IADL scale scores) (Hancock and Larner 2007).
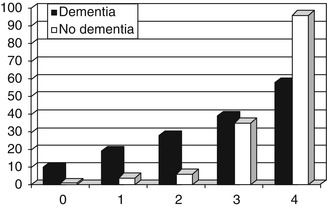

Figure 5.2
Distribution of 4-IADL Scale scores vs. diagnosis
The modest results for diagnostic utility of the IADL Scale might be accounted for in part by the fact that most patients in this population achieved high IADL Scale scores (Fig. 5.1) although this was no guarantee of the absence of dementia. It is well attested that such a ceiling effect is best avoided in diagnostic scales. Whether objective assessments accurately record changes in everyday life competence has been noted to relate mainly to the sensitivity of IADL instruments (Nygård 2003). Other investigators have also reported the absence of functional decline, as measured using the IADL, in AD patients (Park et al. 2007). Combining IADL Scale scores with a cognitive measure (ACE-R) has also been examined (see Sect. 5.5.3; Larner and Hancock 2012).
It has been reported that use of the Disability Assessment for Dementia (DAD) scale (Gelinas et al. 1999) may be useful for differentiating FTLD from AD, since the former, especially behavioural variant frontotemporal dementia, has significant impact on activities of daily living (Mioshi et al. 2007). Analysing the data from the IADL study (Larner and Hancock 2008a), mean IADL Scale score for AD patients (n = 122) was 9.7 ± 3.4 and for FTLD patients (n = 11) was 10.5 ± 4.4. The null hypothesis that scores were not different between the two groups was not rejected (t = 0.65, p > 0.5). Likewise, using the 4-IADL score the mean scores (AD 2.8 ± 1.2; FTD 3.0 ± 1.3) were not significantly different (t = 0.47, p > 0.5).
5.2 Behavioural and Psychiatric Scales
Behavioural and psychiatric symptoms (BPSD), as well as cognitive deficits, define the dementia phenotype (American Psychiatric Association 2000; Ballard et al. 2001; Savva et al. 2009). Hence assessment of BPSD may be deemed desirable in the diagnosis and assessment of suspected dementia. Furthermore, the most common differential diagnosis of dementia in patients referred to memory clinics is affective disorder, particularly depression (Roose and Devanand 1999; Berrios and Hodges 2000).
Differentiating between dementia and depression as causes of memory impairment can be difficult on clinical grounds alone. Test instruments which might help with this differential diagnosis, and hence guide treatment options (e.g. cholinesterase inhibitor vs. antidepressant), would therefore be welcome.
5.2.1 Cambridge Behavioural Inventory (CBI)
The Cambridge Behavioural Inventory (CBI) is a short, self-administered, informant questionnaire developed from an analysis of the behavioural and psychiatric features which distinguish AD from FTLD (Bozeat et al. 2000; CBI download may be requested at www.neura.edu.au/frontier/research/test-downloads). CBI is an 81 item, 13 subsection, questionnaire (Box 5.2) in which informants are asked to score various behavioural and psychiatric symptoms subjectively according to a frequency-based intensity scale (for most symptoms minimum = 0, not present; maximum = 4, constantly present; hence possible range of global CBI score = 0–324).
Box 5.2: Item Content of CBI
Memory | 6 items |
Orientation and Attention | 7 items |
Everyday skills | 8 items |
Self care | 7 items |
Mood | 9 items |
Beliefs | 7 items |
Challenging behaviour | 4 items |
Disinhibition | 5 items |
Eating habits | 5 items |
Sleep | 2 items |
Stereotypic and motor behaviours | 11 items |
Motivation | 8 items |
Insight/awareness | 2 items |
Total score | 81 items |
CBI has been shown to have adequate test-retest reliability and convergent validity with the Neuropsychiatric Inventory (NPI; Cummings et al. 1994) in an independent cohort (Nagahama et al. 2006). It has been used qualitatively in drug trials (Deakin et al. 2004). CBI may have clinical utility in differentiating different neurodegenerative disorders (Wedderburn et al. 2008). A revised version of the CBI has also been published (Wear et al. 2008).
The diagnostic utility of CBI has been assessed prospectively in new referrals to CFC and to the Brooker Centre, Runcorn, over an 18-month period (January 2006-June 2007) (Hancock and Larner 2008; Larner 2008a; Larner and Hancock 2008b). Dementia prevalence was higher in this cohort than in other cohort studies reported from these clinics. This was because patients without dementia sometimes attend the clinic without an informant despite receiving written instructions to do so in their appointment letter (“attended alone” sign; see Sect. 3.2.1; Larner 2005a, b, 2009a, 2012a); these individuals were by definition not represented in this cohort.
The results (Table 5.2) showed only modest diagnostic utility for the CBI, none of the parameters reaching the desired levels. For the differential diagnosis of AD and FTLD, the difference between the CBI global scores for patients with AD (n = 79, range 20–239, mean 93.6 ± 53.1) and FTD (n = 11, range 19–216, mean 101.2 ± 56.3) did not reach statistical significance (t = 0.44, p > 0.5) (Larner and Hancock 2008b).
CBI | |
|---|---|
N | 159 |
Prevalence of dementia | 63 % |
M:F (% male) | 86:73 (54) |
Age range in years | 37–97 |
Cutoff | 80/324 |
Accuracy | 0.62 (0.54–0.69) |
Sensitivity (Se) | 0.54 (0.44–0.64) |
Specificity (Sp) | 0.75 (0.63–0.86) |
Y | 0.29 |
PPV | 0.78 (0.69–0.88) |
NPV | 0.49 (0.39–0.59) |
PSI | 0.27 |
LR+ | 2.12 (1.32–3.41) = small |
LR− | 0.62 (0.38–0.99) = unimportant |
DOR | 3.44 (2.15–5.53) |
CUI+ | 0.42 (poor) |
CUI− | 0.37 (poor) |
Based on the CBI symptoms shown (Bozeat et al. 2000) to be most suggestive of AD (memory, orientation and attention, everyday skills; item subtotal = 21, possible CBI subscore range = 0–84) and of FTLD (disinhibition, eating habits, stereotypic and motor behaviours; item subtotal = 21, possible CBI subscore range = 0–84), a CBI ratio subscore (AD:FTLD) was devised (Larner 2008a). The formulation of this ratio was based on the principles used to derive a subscore (VLOM ratio) from the Addenbrooke’s Cognitive Examination (ACE), which is reported to differentiate AD and FTLD based on cognitive features (Mathuranath et al. 2000; see Sect. 4.5.1). A similar ratio may be derived from the Montreal Cognitive Assessment (MoCA VLOM; Sect. 4.9.1). The CBI ratio subscore in the patients recruited from CFC (n = 75) was found to have a maximal diagnostic accuracy (0.85) using a cutoff score of 1 (where <1 = FTLD, ≥1 = AD). Sensitivity and positive predictive value at this cutoff were high, but confidence intervals were large because patient numbers were small (Table 5.3).
Table 5.3
Demographic and diagnostic parameters for CBI ratio subscore for differential diagnosis of Alzheimer’s disease and frontotemporal dementia (Larner 2008a)
CBI ratio subscore | |
|---|---|
N | 75 |
Prevalence dementia | 64 % |
M:F (% male) | 39:36 (52) |
Age range in years | 39–85 |
Cutoff | 1 |
Accuracy | 0.85 (0.74–0.95) |
Sensitivity (Se) | 0.95 (0.87–1.00) |
Specificity (Sp) | 0.44 (0.12–0.77) |
Y | 0.39 |
PPV | 0.88 (0.77–0.98) |
NPV | 0.67 (0.29–1.00) |
PSI | 0.55 |
LR+ | 1.70 (0.94–3.07) = unimportant |
LR− | 0.12 (0.07–0.22) = moderate |
DOR | 14.0(7.77–25.2) |
CUI+ | 0.84 (excellent) |
CUI− | 0.29 (very poor) |
CBI has also proved useful in documenting behavioural symptoms in individual cases (Larner 2008b, 2013a; Case Study 5.1).
Case Study 5.1: Clinical Utility of Behavioural Screening Instrument in Diagnosis of Dementia: CBI
Change in personality and decline in activities of daily living developed progressively in a professional woman in her early 40s. There was no past history of medical or psychiatric illness. An empirical trial of antidepressant medications produced no clinical response. The CBI, completed by the patient’s husband, showed evidence for impaired self-care (difficulty self-grooming), mood change (rapid shifts in emotions), change in dietary habits (eating the same food repeatedly), disinhibition (acting impulsively) and stereotyped and motor behaviours (following routines, hoarding, echolalia). These features were suggestive of a diagnosis of behavioural variant frontotemporal dementia. Subsequent neuroimaging studies showed structural and functional changes consistent with this diagnosis (CT and MRI: marked bilateral frontal brain atrophy; SPECT: bilateral frontal hypoperfusion). Neurogenetic testing showed the hexanucleotide repeat expansion in the C9ORF72 gene.
On the basis of these results, CBI global score cannot be recommended as a quantitative bedside test for the diagnosis of dementia in preference to cognitive tests, since its diagnostic utility proved to be only modest with cross-sectional use. However, CBI retains a place in the qualitative evaluation of patient symptoms which may guide appropriate patient management (e.g. as found in individual patients: Larner et al. 2007; Case Study 5.1). The overall benefit of CBI may be in providing a structured behavioural symptom profile rather than a summated behavioural score (Wedderburn et al. 2008).
5.2.2 Patient Health Questionnaire-9 (PHQ-9)
The Patient Health Questionnaire-9 (PHQ-9) is a validated instrument for measurement of the severity of depression (Kroenke et al. 2001). It is a nine symptom depression checklist, with each symptom graded by frequency (score range 0–3) over the preceding 2 weeks (Box 5.3). PHQ-9 scores range from 0–27, with 0–4 adjudged to indicate no depression, 5–9 mild depression, 10–14 moderate depression, and ≥15 severe depression.
Box 5.3: Item Content of PHQ-9
Little interest or pleasure in doing things
Feeling down, depressed or hopeless
Trouble falling or staying asleep or else sleeping too much
Feeling tired or having little energy
Poor appetite or overeating
Feeling bad about self, a failure, have let self or family down
Trouble concentrating (reading, TV)
Moving or speaking so slowly that others have noticed; or fidgety, restless
Thought you would be better off dead, or of hurting yourself
PHQ-9 has proved useful in the recognition of depression in the general population (Martin et al. 2006), in primary care (Gilbody et al. 2007a), and in medical settings (Gilbody et al. 2007b). PHQ-9 may also be sensitive to change over time and following treatment with antidepressants (Löwe et al. 2004, 2006). In the UK general practitioner (GP) Quality and Outcome Framework (British Medical Association 2006), PHQ-9 was one of the recommended measures of depression severity. Its reported use has been encountered in referrals to CFC from primary care (see Table 1.3; Menon and Larner 2011; Ghadiri-Sani and Larner 2014).
The diagnostic utility of PHQ-9 has been assessed prospectively in new referrals to CFC and to the Brooker Centre, Runcorn, over a 10-month period (June 2007-March 2008) (Hancock and Larner 2009a). PHQ-9 proved easy to use, being completed in all cases, although some patients required the assistance of a relative, friend, or other carer. PHQ-9 scores ranged from 0 to 25 (Fig. 5.3). For the demented group, the mode, median, and mean PHQ-9 scores were 0, 2, and 4.1 ± 5.4, respectively; for the non-demented group the mode, median, and mean scores were 0, 3.5, and 7.8 ± 7.9. The mean PHQ-9 scores differed significantly between the two groups (t = 2.80, df = 111, p < 0.01).
Diagnostic utility of PHQ-9 at the optimal accuracy (cutoff 9/27; Table 5.4) was modest. Performance compared unfavourably with a meta-analysis of PHQ-9 for a DSM-IV diagnosis of major depressive disorder (sensitivity 0.80, specificity 0.92, LR+ 10.12, LR− 0.22; Gilbody et al. 2007b).
PHQ-9 | |
|---|---|
N | 113 |
Prevalence dementia | 43 % |
M:F (% male) | 55:58 (49) |
Age range in years | 29–94 (mean 68.3 ± 11.7) |
Cutoff | 9/27 |
Accuracy | 0.62 (0.53–0.71) |
Sensitivity (Se) | 0.86 (0.76–0.96) |
Specificity (Sp) | 0.44 (0.32–0.56) |
Y | 0.30 |
PPV | 0.54 (0.43–0.65) |
NPV | 0.80 (0.67–0.93) |
PSI | 0.34 |
LR+ | 1.52 (1.19–1.95) = unimportant |
LR− | 0.32 (0.26–0.42) = small |
DOR | 4.67 (3.65–5.96) |
CUI+ | 0.46 (poor) |
CUI− | 0.35 (very poor) |
AUC ROC curve | 0.63 (0.53–0.73) |
The PHQ-9 cutoff of 9/27 coincided with the defined test threshold between mild and moderate depression (Kroenke et al. 2001). Dependent on clinical context, this cutoff might be taken as an indicator of the need or otherwise for antidepressant medication. Using this pragmatic threshold, the null hypothesis that the proportion of patients with at least moderate depression did not differ significantly between patients diagnosed with dementia (6/49 = 12 %) and without dementia (26/64 = 41 %) was examined, and rejected (χ2 = 11.3, df = 1, p < 0.01). Hence the relative risk or risk ratio for moderate depression, defined by a PHQ-9 cutoff score of 9/27, in non-demented compared to demented individuals was 3.32 (95 % CI = 1.48–7.43).
The correlation coefficient for PHQ-9 scores and simultaneously recorded Mini-Mental State Examination (MMSE) scores (n = 106) was, as expected, very low (r = 0.01, t = 0.08, df = 104, p > 0.5), since these tests measure different constructs, and likewise for simultaneously recorded ACE-R scores (n = 97; r = 0.12, t = 1.19, df = 95, p > 0.1).
The correlation between PHQ-9 scores and CBI scores was examined in those patients undergoing both tests (n = 50). There was a low positive correlation (r = 0.33; t = 2.40, df = 48, p ≈ 0.02). That the correlation was no better might be anticipated considering the wider coverage of behavioural features, not only depression, in the CBI. Overall performance of PHQ-9 was similar to that of the CBI (compare Tables 5.2 and 5.4). A study in dementia clinics to compare PHQ-9 with the Cornell Scale for Depression in Dementia (Alexopoulos et al. 1988) would seem to be desirable.
5.2.3 Addenbrooke’s Cognitive Examination (ACE) and Addenbrooke’s Cognitive Examination-Revised (ACE-R)
Although the Addenbrooke’s Cognitive Examination (ACE; Mathuranath et al. 2000; see Sect. 4.5) and its revision (ACE-R; Mioshi et al. 2006; Sect. 4.6) are not behavioural and psychiatric scales (Davies and Larner 2013), nonetheless they are included here because it has been reported that ACE scores may be able to distinguish dementia and affective disorder (Dudas et al. 2005). Patients with dementia scored lower than individuals with “pure” affective disorder, with low scores on the memory domain tasks and letter fluency but with preserved category fluency indicating affective rather than “organic” pathology (Dudas et al. 2005). A later study of the Danish translation of ACE challenged this observation, finding great overlap in individual test scores for demented and depressed patients (Stokholm et al. 2009).
In a study undertaken at the Brooker Centre, Runcorn, over a 17-month period (December 2006-April 2008) ACE-R was administered to 119 patients of whom 54 had a final diagnosis of dementia and 19 of pure affective disorder, the remainder having either mixed or no pathology (using the diagnostic categories as per Dudas et al. 2005). Mean ACE-R (and MMSE) scores differed between these groups (Table 5.5) but statistical calculations of group differences were not undertaken (P Hancock, personal communication, 30 June 2008).
Table 5.5
Demographic and diagnostic parameters for ACE-R and MMSE in dementia and pure affective disorder
ACE-R/MMSE | |||||
|---|---|---|---|---|---|
N | 119 | ||||
M:F (% male) | 56:63 (47) | ||||
Mean age (years) | 70.6 ± 9.9 | ||||
n | M:F | Mean age (years) | Mean MMSE | Mean ACE-R | |
Dementia | 54 | 27:27 | 74.2 ± 8.5 | 21.2 ± 3.9 | 60.3 ± 12.1 |
Pure affective disorder | 19 | 8:11 | 60.0 ± 7.3 | 28.9 ± 1.1 | 87.9 ± 9.0 |
Mixed dementia + affective disorder | 6 | 2:4 | 73.0 ± 5.2 | 26.3 ± 1.8 | 73.0 ± 7.1 |
No dementia | 40 | 19:21 | 75.3 ± 11.6 | 27.4 ± 2.3 | 87.8 ± 10.8 |
5.3 Neurovegetative Symptoms
DSM-IV acknowledges that the “multiple cognitive impairments of dementia are often associated with anxiety, mood and sleep disturbances” (American Psychiatric Association 2000:150). It has become increasingly apparent in recent years that sleep is crucial for physiological memory function, perhaps most especially for memory consolidation (Wagner et al. 2004; Siegel 2005; Stickgold 2005).
Abnormal sleep is a feature not only of depression but also of a number of dementing disorders associated with impaired memory function, including Alzheimer’s disease (Bliwise 2004; Petit et al. 2004). Occasional examples of specific sleep signatures in neurodegenerative disease, such as REM sleep behaviour disorder in dementia with Lewy bodies (DLB) and other synucleinopathies (Boeve et al. 2007), may be encountered in patients attending dedicated memory clinics (Larner et al. 2005). The Mayo Fluctuations Questionnaire, which may assist in the diagnosis of Parkinson’s disease dementia (PDD) and DLB, specifically asks about sleep and sleepiness (Ferman et al. 2004; see Sect. 5.4.3).
Sleep-related disorders may present de novo to memory clinics, such as restless legs syndrome (Davies and Larner 2009), shift-work sleep disorder (Davies and Larner 2009; Larner 2010a), and sleep apnoea syndromes (obstructive and central) (Larner and Ghadiali 2008; Lim and Larner 2008). Studies have therefore been undertaken to examine whether use of sleep questionnaires might be helpful in diagnosis and assessment of patients with cognitive complaints.
5.3.1 Pittsburgh Sleep Quality Index (PSQI)
The Pittsburgh Sleep Quality Index (PSQI; Buysse et al. 1989) is a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month period (Box 5.4) to generate seven component scores (range 0–3) and one global score (range 0–21).
Box 5.4: Item Content (Components) of PSQI
Q1. Subjective sleep quality
Q2. Sleep latency
Q3. Sleep duration
Q4. Habitual sleep efficiency
Q5. Sleep disturbances
Q6. Use of sleeping medication
Q7. Daytime dysfunction
In the original study, a global PSQI score >5 distinguished good and poor sleepers (sensitivity 89.6 %, specificity 86.5 %). The PSQI has been reported to be a stable measure of sleep quality (Knutson et al. 2006), with high test-retest reliability and construct validity (Gentili et al. 1995; Backhaus et al. 2002). PSQI has proved useful for characterizing sleep disturbances in conditions such as fibromyalgia and post-traumatic brain injury (Fictenberg et al. 2001; Osorio et al. 2006). Poor sleep quality has also been reported to have a possible role in the differential diagnosis of dementia syndromes, specifically of Parkinson’s disease dementia and dementia with Lewy bodies from AD (Boddy et al. 2007). PSQI has been translated into a variety of languages.
The diagnostic utility of PSQI to facilitate clinical differential diagnosis of patients with and without dementia at the initial diagnostic interview has been assessed prospectively in new referrals to CFC and to the Brooker Centre, Runcorn, over a 2-year period (February 2006-February 2008) (Hancock and Larner 2009b). This was based on a clinical impression that non-demented patients attending CFC had poor sleep compared to those with dementia. Global PSQI scores ranged from 0 to 20 (dementia group 0–18, non-dementia group 0–20). The mean global PSQI score in the dementia and non-dementia subgroups was 5.1 (±4.2) and 7.6 (±5.1), respectively, a difference which proved statistically significant (t = 4.64, p < 0.001). Using the PSQI categorisation of good (PSQI ≤ 5) or bad (PSQI > 5) sleep quality, of the good sleepers (n = 165), 62 % had dementia and 38 % were not demented, whilst for the bad sleepers (n = 145) the corresponding figures were 36 and 64 % respectively (Z = 4.23, p < 0.01). Hence, the relative risk or risk ratio for poor sleep quality, defined by a PSQI cutoff score of ≤5/21, in non-demented compared to demented individuals was 1.79 (95 % CI = 1.38–2.31)
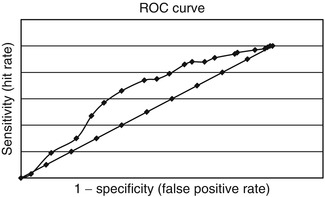
Diagnostic utility of PSQI was modest, with both sensitivity and specificity for the diagnosis of dementia <0.7 (Table 5.6). Area under the ROC curve was 0.64 (Fig. 5.4).
PSQI | |
|---|---|
N | 310 |
Prevalence dementia | 50 % |
M:F (% male) | 158:152 (51) |
Age range in years | 29–97 (mean 66.9 ± 13.0) |
Cutoff | 5/21 |
Accuracy | 0.63 (0.58–0.69) |
Sensitivity (Se) | 0.66 (0.59–0.74) |
Specificity (Sp) | 0.60 (0.52–0.68) |
Y | 0.26 |
PPV | 0.62 (0.55–0.70) |
NPV | 0.64 (0.56–0.72) |
PSI | 0.26 |
LR+ | 1.66 (1.32–2.08) = unimportant |
LR− | 0.56 (0.45–0.70) = unimportant |
DOR | 2.97 (2.38–3.71) |
CUI+ | 0.41 (poor) |
CUI− | 0.38 (poor) |
AUC ROC curve | 0.64 (0.58–0.70) |
Analysis using a suggested 3-factor scoring model (Cole et al. 2006) encompassing sleep efficiency (Q3 + Q4; score range 0–6), perceived sleep quality (Q1 + Q2 + Q6; score range 0–9), and daily disturbance (Q5 + Q7; score range 0–6) did not result in better accuracy than the global PSQI score (best accuracy: sleep efficiency 0.62 at cutoff 2; perceived sleep quality 0.63 at cutoff 1; daily disturbance 0.57 at cutoff 1).
A subgroup of patients completed PHQ-9 (see Sect. 5.2.2) as well as PSQI (n = 96). PSQI scores and PHQ-9 scores showed a moderate positive correlation in both the dementia group (n = 44; r = 0.53, t = 4.01, p < 0.001) and the non-dementia group (n = 52; r = 0.62, t = 5.64, p < 0.001).
5.3.2 Sleep Disorders Inventory (SDI)
The importance of sleep disorders in established dementia syndromes has been increasingly recognised. Nocturnal sleep disturbance and abnormal daytime sleepiness may predict accelerated functional decline in dementia patients, as well as contributing to caregiver distress. Carers may find sleep disruption more challenging than memory problems. Sleep related problems are more common and severe in the advanced stages of dementia, although they may commence early in the disease course, and increase the likelihood of institutionalisation (Moe et al. 1995). Cholinesterase inhibitors may improve memory at least in part through beneficial effects on sleep (Schredl et al. 2001).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree