Attention-Deficit/Hyperactivity Disorder
Lacramioara Spetie M.D.
L. Eugene Arnold M.D.
Definition
Attention deficit/hyperactivity disorder (ADHD) is a syndrome of inattention, distractibility, restless overactivity, impulsiveness and other deficits of executive function. It involves impairment of the ability to “plan your work and work your plan.” It can be manifested in any of 18 official DSM-IV symptoms (Table 5.2.1.1) and can be full-expression (combined type) or partial expression (inattentive or hyperactive-impulsive types). It is not necessary to have all the symptoms to qualify for the diagnosis. Six of the nine inattentive symptoms are required for diagnosis of inattentive type and six of the hyperactive-impulsive for diagnosis of hyperactive/impulsive type, with six of each list for combined type (1). Thus, it is possible for two patients to meet diagnostic criteria with no symptoms in common and it is even possible to have the same subtype with only half the symptoms in common. This leads to wide variability in presenting problems and severity, which is further complicated by common associated symptoms such as irritability, boredom, and impaired social skills, and by psychiatric comorbid diagnoses.
TABLE 5.2.1.1 DSM-IV DIAGNOSTIC CRITERIA FOR ATTENTION DEFICIT HYPERACTIVITY DISORDER | ||
|---|---|---|
|
The Attention Deficit Hyperactivity Disorder Concept and Terminology
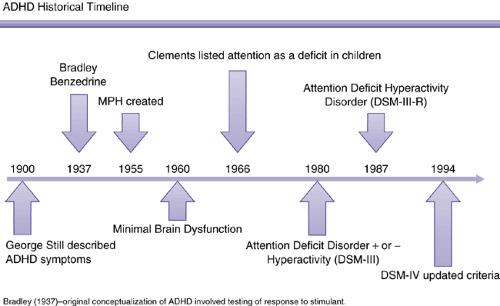
As early as the mid-nineteenth century, the problems of inattentiveness and overactivity in children were recognized by Heinrich Hoffman (2) in the moralistic children’s book Slovenly Peter, which featured the characters Fidgety Phil and Harry Who Looks in the Air. In the early twentieth century Still’s disease was recognized as the behavioral sequelae of viral encephalitis. The behavioral constellation, particularly the overactivity and impulsiveness, were therefore considered “minimal brain damage,” which morphed over time
into “minimal brain dysfunction” as diagnosticians realized it was not possible to find evidence of actual brain damage in most cases (at least not with the technology available through the 1970s). Other descriptive terms used by the second half of the twentieth century included hyperkinesis, hyperkinetic syndrome, hyperactivity, hyperactive-impulse disorder, psychoneurologic integration deficit, and pseudoneurosis.
into “minimal brain dysfunction” as diagnosticians realized it was not possible to find evidence of actual brain damage in most cases (at least not with the technology available through the 1970s). Other descriptive terms used by the second half of the twentieth century included hyperkinesis, hyperkinetic syndrome, hyperactivity, hyperactive-impulse disorder, psychoneurologic integration deficit, and pseudoneurosis.
The second edition of the Diagnostic and Statistical Manual (3) recognized the syndrome for the first time as an official diagnosis, calling it “hyperkinetic reaction,” based on the then-prevailing psychodynamic philosophy that mental disorders were always reactions to some stressor. The term “hyperkinetic disorder” is still used in the current International Code of Diseases (4). Recognizing that the cause of most mental disorders is more complex than a reaction to stress, the DSM III (5) changed in 1980 to descriptive phenomenological terms without causal implication. By that time the importance of inattentive symptoms had been recognized, leading to the appellation “attention deficit disorder,” which could be diagnosed either without (ADD) or with hyperactivity (ADDH). The term ADD is still used by many to refer to the inattentive subtype, and is preserved in the names of such support/advocacy groups as Attention-Deficit Disorder Association (ADDA) (6) and Children and Adults with ADD (ChADD) (7). DSM-III-R (8) added overactivity back to the name of the disorder via the term “attention deficit/hyperactivity disorder (ADHD).” This name was retained by DSM-IV (1), although the symptom list changed somewhat, being expanded from 14 to 18 symptoms and being split into two lists of nine each (Figure 5.2.1.1).
The ICD 10 criteria for Hyperkinetic Disorder (HD) are more restrictive than the DSM IV criteria in that all three symptom clusters of inattention, hyperactivity and impulsivity should be present and pervasive across settings and that the presence of anxiety or mood disorder is in itself an exclusion criterion. ICD 10 also includes the diagnosis of hyperkinetic conduct disorder (HKD), which would be roughly the same as the DSM equivalent of ADHD combined type with comorbid conduct disorder.
Epidemiology
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood onset psychiatric disorders, affecting 5–12% of children worldwide. ADHD is a costly public health concern (9) since it can cause significant impairment in functioning that interferes with normal development and all areas of functioning in patients of all ages.
Epidemiological studies of ADHD addressing issues of frequency, distribution, determinants, comorbidity, long-term outcome, and impact of treatment have been complicated by several problems. The first is integrating the different types of information from various sources required to make the diagnosis. A related problem is how to correct for the subjective nature of the information provided by the informants. The diagnosis could be easily overestimated if the information obtained does not include the level of impairment caused by the symptoms reported (10). Another problem is the difficulty integrating epidemiological information obtained over time due to the ongoing refinement in the diagnostic criteria for ADHD. The current DSM IV criteria allow the inclusion of more females, preschoolers, and adults presenting with significant impairment but who would have been otherwise excluded (11). The definition of ADHD may continue to flux over time, as experts now consider more age-specific thresholds for symptom counts.
Further, there are differences between the DSM IV criteria used to make the diagnosis in the United States and the ICD-10 criteria ordinarily used to make the diagnosis in Europe and other parts of the world, although many investigators worldwide use the DSM-IV system. Because of the difference in stringency of definition, DSM IV ADHD is more prevalent than ICD HKD, a finding that could be easily misinterpreted to show that ADHD is more common in countries using DSM criteria than in countries using ICD-10 criteria (12). A recent reanalysis of the data of the National Institute of Mental Health (NIMH) Multimodal Treatment Study of children with ADHD (the MTA) found that only 145 of the 579 children who had DSM-IV combined type ADHD also met criteria for ICD-10 HKD (13). A further complication is whether all the inattentive type cases are detected in a given study.
However, when DSM-IV criteria are applied to the epidemiological information obtained in various international studies, the prevalence in the United States and worldwide is similar, 5–12%, including fairer representation of females and inattentive type, which may have been underappreciated in earlier lower estimates, such as in the DSM-IV (1) estimate of 3–5%. The male to female ratio is about 2:1 in epidemiologically discerned samples, in contrast to 3–5:1 and even up to 9:1 in mental-health clinic samples. Females present more often with less disruptive symptoms, more attention problems and more internalizing problems such as depression and anxiety (14), while boys present with more
disruptive behavior leading to clinical referral. Male sex, low socioeconomic status, and young age are associated with a higher prevalence of ADHD.
disruptive behavior leading to clinical referral. Male sex, low socioeconomic status, and young age are associated with a higher prevalence of ADHD.
In preschoolers the hyperactive subtype is more common and the prevalence may vary from a low of 2% in the primary care setting to a high of 59% in a child psychiatry clinic (15). The symptoms related to hyperactivity tend to decrease with age. In intermediate elementary school-age children the combined type has been more frequently diagnosed, while the inattentive type was more common in middle and high school. As many as 60% of the childhood cases continue to have some symptoms as adults.
Etiology
The etiology of ADHD is complex and most likely includes genetic and environmental factors.
Genetics
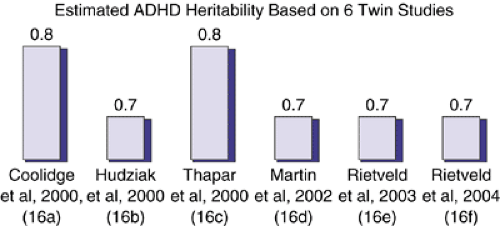
ADHD is a genetically predisposed disorder that does not follow the Mendelian patterns of inheritance and that is also phenotypically complex. The early genetic studies relied on a categorical diagnosis of ADHD without taking into account differences in the manifestation of the various components of the illness and the currently recognized subtypes, making their results difficult to interpret. Recent studies have shown that ADHD is strongly genetically influenced and is transmitted in families. Extensive data gathered by twin and adoption studies have consistently yielded an estimated heritability of about 75% that has not changed over the past 30 years (Figure 5.2.1.2) (16a-f).
Developments in human genetics, including the completion of the sequencing of the human genome, the availability of powerful technology for genomic analysis, and the generation of new analytical and bioinformatics tools, have led to significant progress in understanding the genetics of many neurodevelopmental disorders, including ADHD. Recent genetic studies have been using endophenotypes as tools to detect the effects of individual genes. Endophenotypes are phenotypes that are assumed to be less complex in presentation and etiology than signs and symptoms of the clinical disorder but are still influenced by one or more of the same susceptibility genes as the disorder. Neuropsychological measures of inhibitory control, impairments in state regulation, and delay aversion are considered potential candidates for ADHD endophenotypes (17,18). Other methods focus on distinct components or clusters of symptoms of the complex phenotypes that may be heritable and may characterize phenotypically homogenous groups of individuals.
So far there have been three genome-wide linkage studies, and each of them identified different chromosomal regions shared more often than expected by chance among ADHD family members. These regions have included 5p12, 10q26, 12q23 and 16p13 (19); 15q15, 7p13 and 9q33 (20); and 8q12, 11q23, 4q13, 17p11, 12q23, and 8p23 (21).
Association studies of candidate genes have looked for evidence that certain biologically relevant candidate genes may influence the susceptibility to ADHD. Case-control designs compare allele frequencies between patients with ADHD and non-ADHD control subjects, while family-based designs compare the alleles that parents transmit to ADHD children with those they do not transmit. If an allele increases the risk for ADHD, it should be more common among the transmitted alleles than the nontransmitted alleles. The data obtained in both study designs can be analyzed to derive odds ratio (OR) or relative risk (RR) statistics that assess the magnitude of the association between the risk alleles and the diagnosis of ADHD. An OR or RR of 1.0 indicate no association, greater than 1.0 indicates that the allele increases the risk for ADHD, and those less than 1.0 indicate that the allele decreases the risk for ADHD. The most studied candidate genes for ADHD are listed in Table 5.2.1.2.
Structural, Functional, and Electrophysiological Findings in the Brain
Structural Findings in ADHD
Similarities between the symptoms of attention deficit hyperactivity disorder and symptoms observed in certain neurological patients following damage to the prefrontal cortex (22) have prompted researchers to theorize that brain structural abnormalities may be at the root of ADHD symptomatology. Furthermore, neurocognitive studies of patients with ADHD also identified patterns of executive dysfunction in patients with ADHD that are thought to reflect abnormalities in the functioning of the prefrontal cortex, therefore supporting the hypothesis of an alteration of the prefrontal cortex neuroanatomy in ADHD (23,24,25). The robust symptom response to psychostimulant drugs that target the dopaminergic system that is very well represented in the prefrontal cortex further supported this theory (26). With the introduction of totally automated imaging methods it has been possible to identify more widespread volumetric and cortical changes.
The most consistent structural brain imaging findings in children with ADHD have been significantly smaller volumes in the dorsolateral prefrontal cortex, caudate, pallidum, corpus callosum, and cerebellum (Table 5.2.1.3). There is only one structural brain imaging study in adults (27). Most of the studies to date have had low power (using rather small samples) and have differed widely in the way they address other confounding factors such as the influence of gender, comorbidity, medication status, perinatal complications, DSM subtype, etc. One of the most comprehensive longitudinal case-control imaging studies was of 152 children and adolescents with ADHD (age range 5–18 years) and 139 age- and sex-matched controls (age range 4.5–19 years) completed at the National Institute of Mental Health (NIMH) from 1991 to 2001. The patients with ADHD had significantly smaller brain volumes on the initial scan in all regions (total cerebrum; cerebellum; gray and white matter for the four major lobes: frontal, temporal, parietal and occipital); unmedicated children with ADHD had significantly smaller total cerebral volumes and significantly smaller total white matter; the volumetric abnormalities persisted with age in total and regional cerebral measures and in the cerebellum, except for the caudate nucleus volumes that were initially abnormal for patients with ADHD but lost the diagnostic difference from controls during adolescence; developmental trajectories for all structures except the caudate remained roughly parallel for patients and controls during childhood and adolescence and are unrelated to stimulant treatment (28).
TABLE 5.2.1.2 CANDIDATE GENES FOR ADHD | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 5.2.1.3 STRUCTURAL BRAIN ABNORMALITIES IN ADHD | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Twin studies using structural neuroimaging suggest that the volume of brain regions relevant to ADHD (subcortical and cortical volumes, left and right neocortex, variation of cerebellar volume) is under significant genetic control and might be used to define neuroimaging ADHD endophenotypes (17).
Brain Functional Abnormalities
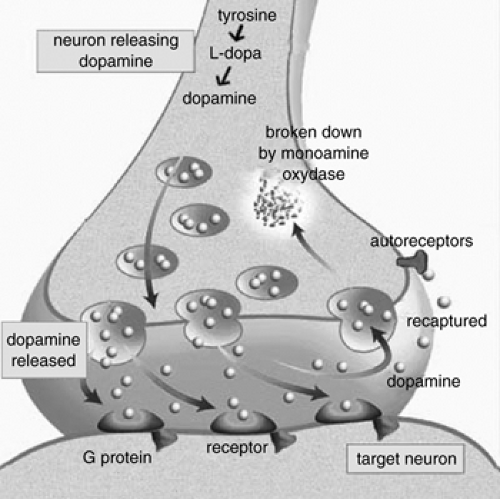
New brain imaging techniques, including single photon emission computed tomography (SPECT); positron emission tomography (PET); functional MRI (fMRI) and proton magnetic resonance spectroscopy (PMRS), have made it possible to obtain dynamic measures of brain metabolism at rest and during certain cognitive tasks. Most studies have found abnormalities in cerebral activation in ADHD, with a hypoperfusion of frontal and possibly striatal areas (Table 5.2.1.4). The studies looking at brain function during tasks that challenge the brain inhibitory control also show deficits in the activation of the brain inhibitory control area in the frontal and striatal regions. Because of the important role dopamine (DA) and the dopamine transporter (DAT) seem to have in the pathophysiology and response to treatment in ADHD, several imaging studies using ligands highly selective for the dopamine transporter sites have studied their density in ADHD subjects compared to controls. These studies consistently found an increase in the DAT binding in the striatum of ADHD subjects compared to controls. Several studies also showed a normalization of this brain function following treatment with methylphenidate.
Such functional investigative tools have opened a window on the dynamic nature of ADHD and started to elucidate the flexible and bidirectional causal relationship between brain structure, its neurochemistry, and brain function. Multiple lines of evidence support the role of dopamine in the etiology and the response to treatment (Figure 5.2.1.3). The dopamine circuits are influenced by inputs from multiple areas of the brain involving other neurotransmitter systems, including norepinephrine and serotonin.
Table 5.2.1.5 (39,30) presents one of the theories explaining the abnormal dopamine transporter density in ADHD subjects and the response to methylphenidate.
Electrophysiological Studies
Electroencephalograms (EEG) provide information about the background electrical activity of the brain with good temporal resolution but poor spatial resolution. EEG studies of patients with attention deficit hyperactivity disorder (ADHD) were first done as early as 1938 (31) when they included mostly qualitative EEG studies that used visual evaluation of paper recordings of the EEG. With the advance of computer-aided spectral analysis, new approaches have been used to evaluate EEG characteristics, including quantitative EEG studies; waveform amplitude studies; power studies; ratio coefficients studies; and coherence studies. Most studies consistently found elevated levels of slow wave activity in comparison to normal children, with the most reliable measure being the relative theta power and reduced amounts of relative alpha and beta waves.
Several studies have looked at EEG as a diagnostic tool and reported that the theta/beta ratio could discriminate ADHD subjects from control subjects with sensitivity and specificity (32,33,34,35). Several models of ADHD and ADHD subtypes have been proposed based on the EEG studies: the Maturational Lag Model; the Developmental Deviation Model; and the Hypoarousal Model (Table 5.2.1.6). All models fail to account for the complex clinical presentation in ADHD.
Event-Related Potentials
Event related potentials (ERPs) provide information about the brain electrical activity underlying sensory and cognitive brain processes in response to stimuli. The small subject numbers, the use of different types of task performance indicators and reward systems, and other methodological flows have made the results of most of the studies to date difficult to interpret (36).
With the development of molecular genetics, attempts have been made to identify electrophysiological endophenotypes of ADHD. A metaanalytic review of twin studies of electrophysiological measures indicates that genetic factors contribute significantly to both EEG and ERP measures, with significant heritability scores for the EEG alpha power and alpha peak frequency and ERP P3 amplitude (37). More studies are needed to refine such endophenotypes.
Special Etiological Subgroups
Because ADHD is a phenomenological diagnosis rather than etiologic, there can be various causes manifested through the same symptom constellations. Genetic predisposition, of course, is a major cause, but genes can exert their influence only through interaction with the environment (and other genes). The pathogenetic mechanisms for expression of various genes in interaction with various environments— physical, chemical, nutritional, familial, social— can vary widely from one individual to another. The multiple allele polymorphisms described above allow for multiple environmental sensitivities or special environmental needs, and there are probably further polymorphisms not yet discovered. For example, there appears to be a small subgroup of children with ADHD who have some kind of food or food additive sensitivity demonstrated in placebo-controlled studies (38,39,40). Another very small subgroup has thyroid abnormality intimately linked to ADHD symptoms (41,42). Heavy metal poisoning can cause ADHD symptoms, and at least one study suggested that in cases of
lead toxicity, ADHD symptoms improve as much (or more) with deleading as with a stimulant drug (43). It is possible that because of genetic differences in enzymes and other metabolic features, some individuals may be more sensitive to heavy metal poisoning than others. Some anticonvulsants can make ADHD symptoms worse. Many authors believe that thresholds of vitamin and mineral requirements vary from person to person, so that some may be more susceptible to borderline deficiency symptoms.
lead toxicity, ADHD symptoms improve as much (or more) with deleading as with a stimulant drug (43). It is possible that because of genetic differences in enzymes and other metabolic features, some individuals may be more sensitive to heavy metal poisoning than others. Some anticonvulsants can make ADHD symptoms worse. Many authors believe that thresholds of vitamin and mineral requirements vary from person to person, so that some may be more susceptible to borderline deficiency symptoms.
TABLE 5.2.1.4 FUNCTIONAL NEUROIMAGING STUDIES OF ADHD | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree