FIGURE 14-1 Attention effects on early somatosensory responses in humans. Left panel: Attention spatially directed toward one hand enhances early somatosensory responses to all stimuli addressed to this same hand. The attention effect involves sensory networks in areas BA1-2 of S1, in the crown of the postcentral gyrus, at 25–50 ms poststimulus. Right panel: Selective attention to the hand stimulated by nociceptive inputs (delivered via a laser beam) also enhances the earliest sensory responses from S1 and S2, recorded from subdural electrodes. Note that the earliest somatosensory responses to non-noxious (Aβ) input arises exclusively from S1 (left panel), while in case of nociceptive Aδ-spinothalamic input (right panel) sensory responses are simultaneous in S1 and the opercular (parasylvian) cortex, including S2. (Data from Garcia-Larrea et al. [26] and Ohara et al. [64], reproduced with permission.)
Attention-related changes in the processing of noxious stimuli are not restricted to sensory cortices: associative, multimodal, and supramodal regions also change their activity patterns under conditions in which attention is modulated. In 1999, Peyron et al. [68] used PET to show that selective attention to pain stimuli entailed changes in a widely distributed cortico-subcortical network, including thalamus and sensory areas, but also the anterior cingulate (ACC), posterior parietal (PPC), and dorsal prefrontal (DLPFC) cortices (Fig. 14-2). Distraction from pain was shown to reduce the activity of these areas, in correlation with a decrease in subjective pain reports, but distraction also showed enhanced responses in regions involved in descending pain control, in particular the perigenual cingulate (pgACC), orbitofrontal cortex (OFC), and periaqueductal gray (PAG) matter [91, 96]. By the turn of the century it had become clear that (i) attended and unattended noxious stimuli were processed differently in sensory areas, (ii) that this occurred before the generation of a perceptual representation of the stimulus, and (iii) that these early effects triggered subsequent changes in higher-order areas subserving conscious perception. Since the decision to attend is deliberate, such mechanisms are defined as “top-down,” whereby an a priori resolution to direct attention, triggered by high-order networks, influences lower-level sensory responsiveness and amplify sensory transmission. The enhanced output from sensory cortices influences in turn associative and multimodal evaluative networks, hence modulating the perception of the painful event. The use of attention/distraction procedures to control pain is being increasingly adapted to clinical settings, in particular with the advent of virtual reality procedures [33].†
A Paradoxical Analgesic Effect of Sustained Attention?
While attending to a noxious stimulus most often tends to enhance pain sensations, it can also, under certain circumstances, minimize pain-related perceptions. This most often relates to the unpleasantness of the pain stimulus (i.e., the pain-related “suffering”), rather than the perception of its intensity. For instance, the emotional distress reported during the cold pressor test (immersing the hand in ice-cold water) decreased when subjects attended deliberately to their bodily sensations throughout the test [50, 56], and this was found to be more effective to alleviate pain than distraction procedures [41]. These surprising effects, demonstrated more than 20 years ago, may be relevant to understand a number of recent coping strategies and therapies for chronic pain, which are based more on analysis and acceptance of pain characteristics than on simple distraction from them [57]. For instance, recent research is putting much emphasis on “meditation-like” techniques of pain control that are not based on distraction, but rather on deliberate focusing on the moment-to-moment characteristics of bodily events (see Chapter 8). Functional imaging techniques in subjects receiving painful stimuli while practicing such techniques have provided novel and intriguing data. Notably, while sensory responses to painful stimuli were enhanced in sensory cortices (S1, S2, posterior insula), as expected from a sustained attention condition, reduced activity was observed in high-order executive, evaluative, and emotion-related areas such as the dorsolateral prefrontal cortex (DLPFC), the amygdala, and the hippocampus, concomitant with a significant decrease in pain unpleasantness [32, 54]. These results indicate that it is possible to develop “peculiar” states of attention to body events which, instead of inducing a joint increase of sensory and evaluative networks (as is common in selective attention) are on the contrary able to dissociate them, setting up a state of increased sensory, but decreased executive and affective processing and prompting a reduction in the unpleasantness of the pain experience. Such dissociation is reminding of that observed during altered states of consciousness such as hypnosis ([75], and see Chapter 7), and of the dissociation between sensory and evaluative networks induced by emotional stimuli, discussed below in this chapter.
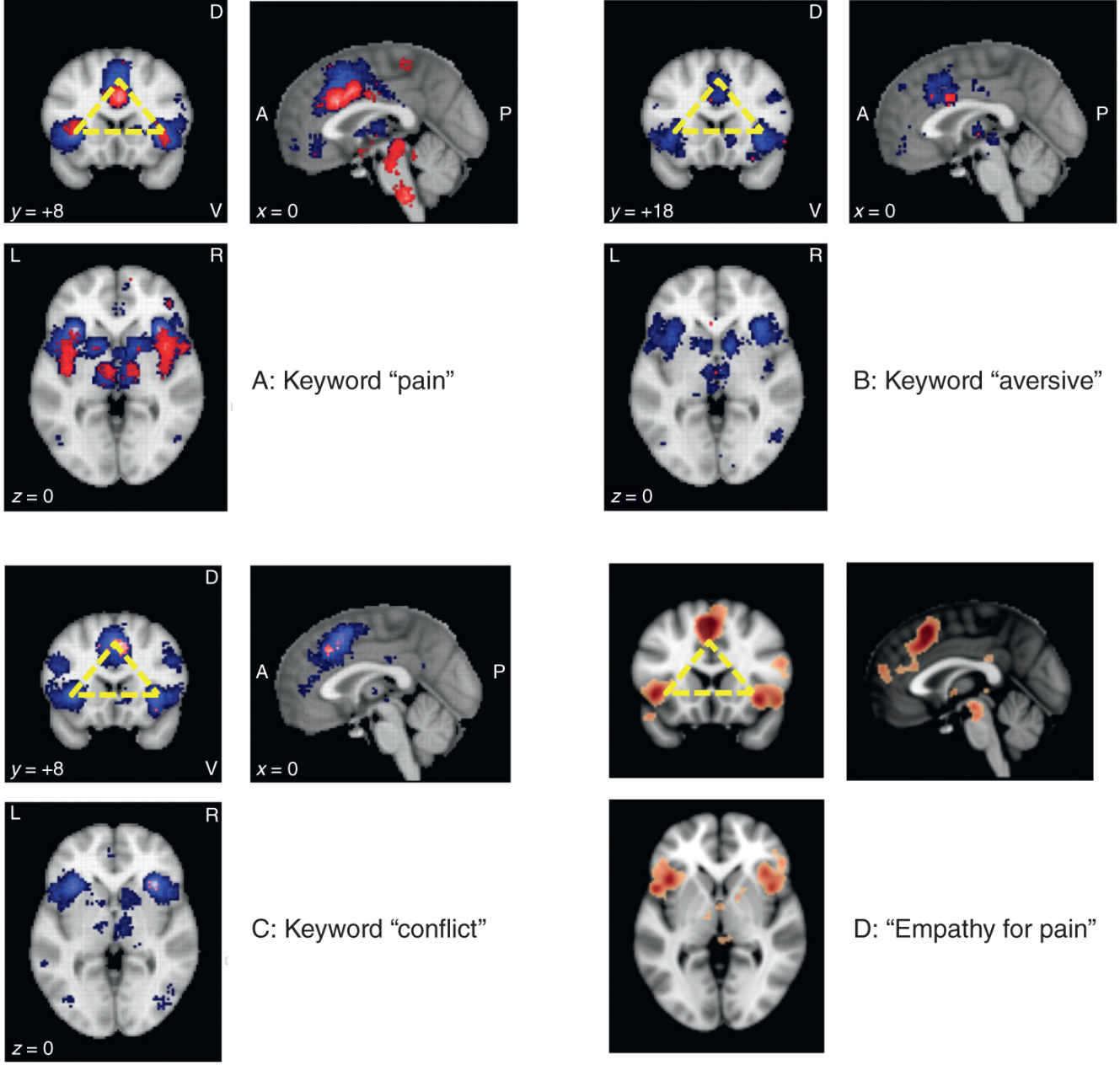
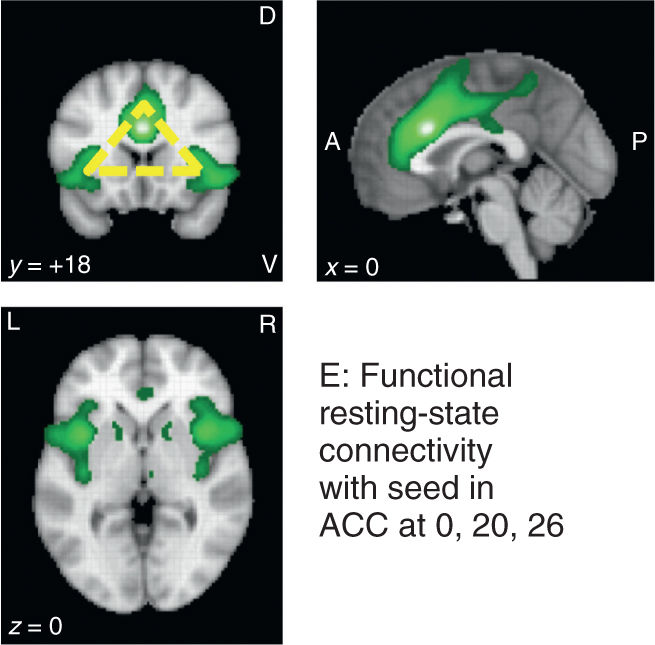
FIGURE 14-2 Joint activations of the dorsal part of the anterior cingulate cortex (ACC) and the anterior part of the insula cortex (AI), known to be multimodal hubs receiving input from sensory and limbic systems, have been associated with a number of different processes. Panel A: activations for “pain” are quite robust as shown by term-based meta-analyses using Neurosynth (www.neurosynth.org; 420 studies, 20,123 activations, threshold = 5). Note that both forward (red) and reverse (blue) inference algorithms show the typical “triangle” activation patterns (top left panel; yellow). Panels B and C: the ACC-AI “triangle” is found for several other keywords implicating emotional relevance, including “aversive” (192 studies, 6602 activations, threshold = 5) and “conflict” (273 studies, 9092 activations, threshold = 5), and is also observed during “empathy for pain”/vicarious pain paradigms (Panel D: modified from: www.neurovault.org; image 12017; original study by Bzdok et al., Brain Struct Funct 2012). Not surprisingly, these two areas show important functional connectivity (Panel E: www.neurosynth.org; resting-state data of 1000 studies; seed placed at 0, 20, 26), supporting the numerous data sets showing that they respond as a functional unit. A, anterior; P, posterior; D, dorsal; V, ventral; L, Left; R, right.
Anticipation Can Be Analgesic or Hyperalgesic
Anticipation and selective attention are tightly associated, anticipation relating strictly to the period when pain is expected but not yet present. Apart from such phenomenological considerations, the mechanisms involved in attention to, and anticipation of, pain largely overlap, and single unit experiments often found it difficult to disclose whether neural responses were associated with the animal’s attention toward or with anticipation of a noxious stimulus [78].
Experiments that selectively manipulate pain anticipation allow assessing brain activity in the absence of actual pain, and hence can dissociate anticipative responses from those involved when a nociceptive stimulus is present. Regions activated during anticipation of pain involve the ACC, anterior insula, and even somatosensory areas, at sites only slightly different from those activated during the processing of actual pain [10, 72]. The electrophysiological concomitants of anticipatory processes (a slow negative potential termed “contingent negative variation”) were also found to involve generators in the mid- and rostral ACC, dorsolateral frontal, and sensory cortices [6]. The magnitude of anticipatory responses increases when the anticipated stimulus is of noxious or aversive nature, and prestimulus negativity to noxious stimuli also increases when they are actively anticipated [46].
Networks involved in pain anticipation and pain processing partially overlap: in humans most sensory and cingulate regions activated during pain anticipation were also activated by somatosensory stimulation [73], and in the macaque half of ACC neurons activated during anticipation of a noxious stimulus were also activated in response to the actual stimulus [42]. Also, in close relation with what occurs during selective attention, anticipation of a noxious stimulus enhances activity within primary somatosensory areas, as if “preactivating” them before stimulus occurrence [73, 74].
Anticipation of pain can either exacerbate or decrease the pain experience, the result greatly depending on the predictable/unpredictable nature of the expected pain stimulus, and the control the subject can exert onto it. In rodents, stimuli predicting with certainty a noxious stimulus consistently decrease the pain behaviors (reviewed in reference [70]), and similar effects have been shown in humans, including in clinical settings, where reliable information about the characteristics of the approaching stimuli decreases clinical pain (reviewed in reference [90]). In animal experiments, anticipation-related analgesia has been linked to a “fear response,” involving activation of the rostralmost cingulate cortex and upper brain stem structures (PAG) triggering descending pain control. The rostral ACC is indeed recruited in humans during pain anticipation [10, 72] and, in many other contexts, its activation has been shown to correlate negatively with perceived pain [67, 73, 74, 96]. Therefore, certainty and cognitive control of the anticipated stimulus may trigger analgesic mechanisms through a common final descending pathway, which is also activated during other analgesic situations including the “freezing” reaction [24], active distraction [91], placebo analgesia, [66] and even neurostimulation procedures of pain control [67]. The neurochemical mediation of anticipation-related analgesia has been suggested to be opioidergic in the short-term, while non-opioidergic systems may activate after extended exposure [51]. Besides fear-related analgesia, sophisticated processes of cognitive control are certainly also active in humans, in whom interaction between high-order cognition and emotional drives are ever present [63].
In contrast with the above, anticipation of aversive stimuli that are unpredictable (i.e., of unknown intensity or aggressiveness) tends to enhance the painful experience [70, 71]. Uncertain expectation of a painful stimulus can amplify perceived unpleasantness and brain responses even to nonpainful stimuli [83]. This effect has been mostly associated with anticipatory anxiety, which enhances pain ratings and participates to clinical pain chronification. Brain networks activated during the expectation of certain versus uncertain pain events may be partly different: uncertain expectancy inducing anxiety has been associated with the ventromedial prefrontal cortex, hippocampal formation, and ventral striatum [34, 38, 70]. Results diverge regarding the direction of changes (i.e., activation vs. deactivation) in these areas, and it has been suggested that enhanced activity in ventromedial prefrontal cortex appears when uncertainty regards the type of upcoming stimulus (e.g., painful or not), whereas activity tends to abate when uncertainty concerns the intensity of the expected stimulus [70]. Such conceptual constructions appear, however, somewhat optimistic regarding the capacities of ventromedial prefrontal networks to “calibrate” the characteristics of upcoming stimuli. A simpler explanation suggests that the magnitude of ventromedial frontal activation during anticipation relates to the intensity of anticipatory anxiety [81]. This explanation appears more parsimonious, and consistent with the role of ventromedial prefrontal cortex in the control of emotions (see reference [63]). Activity in ventromedial frontal networks during anticipation might therefore reflect a “hardwired” reaction that restrains the anxiety state.
The Other Way Round: Automatic (“Bottom-Up”) Orienting to Pain
Any intruding or behaviorally relevant sensory stimuli can drive attention without any previous deliberate decision from the subject. Such stimulus-driven, automatic mode of attention is known as “ascending” or “bottom-up.” It reflects the orienting component of the arousal reaction (c.f. Chapters 1 and 2 in this Volume) and its adaptive value for survival is evident, as failure to orient to crucial stimuli may lead to serious damage or death.
The immediate behavioral relevance of painful stimuli makes them intrinsically attention-capturing. When confronted to several concurrent sensory stimuli, subjects tend to direct attention preferentially toward that of highest intrusiveness [48], and this enhances the subjective perception of intensity and unpleasantness [61]. Importantly, the intruding effects of pain are decreased when the noxious stimuli is delivered while subjects are engaged in a concurrent demanding task [64], which entails decrease in its subjective intensity (an effect known for centuries, and used therapeutically both by folk and academic medicine). It has been argued that enhanced bottom-up orienting, together with failure to modulate noxious perception by cognitive control, may contribute to chronification of pain syndromes such as migraine [15].
Intruding painful stimuli induce rapid shifts of orienting systems involving mid and anterior cingulate (MCC, ACC), partly reflected in positive-polarity waves culminating about 400 ms following nociceptor activation [48]. Functional imaging has suggested that the specific regions within the ACC mediating deliberate (top-down) and automatic (bottom-up) attention may not be the same. Peyron et al. [68] compared the ACC responses during sustained attention to thermal painful stimuli, versus those obtained when a distracted subject was occasionally disrupted by unpredictable painful stimuli. The ACC sectors activated during sustained attention corresponded to rostral area BA32, while disrupting painful stimuli activated a more caudal and posterior ACC region (area BA24). Similar results were reported using a different setting by Davis et al. [14] who compared ACC activity while subjects received occasional painful stimuli versus a sustained attention task of word generation. Again, the ACC loci activation were confined to BA24 in response to (intruding) painful stimuli, but more rostral and superior (BA32) during the sustained attentional task, underscoring the functional bias of posterior sections of the ACC (BA24 and MCC) as systems mediating bottom-up orienting reactions.
Back to Real Life: Bottom-Up and Top-Down Mechanism Acting Together
While experimental settings may allow to disentangle the system structure underlying top-down and bottom-up mechanisms, real-life conditions most often engage both systems, the resulting experience stemming from their coordinated or opposed activity. Any unexpected pain stimuli will drive automatically attention to the body region affected (bottom-up), but this will also induce a top-down reaction of deliberate orienting toward the same region, via frontoparietal descending influences that will increase the sensory gain of primary areas and possibly also the thalamus [39], hence promoting arousal and anticipation toward potential new stimuli. The corollary is that in real situations bottom-up and top-down systems continuously interact, and that the resulting perception can only be predicted by adequately combining the joint contribution of ascending and descending systems. The fact that different modes of anticipation and sustained attention can lead to dissimilar, sometimes opposite experiential consequences explains why attention-based pain modulations are so difficult to reproduce consistently in the clinics. Notwithstanding such difficulties, attention/distraction procedures to control pain have proved useful in different clinical settings, in particular with the recent advent of virtual reality techniques [33].
EMOTION AND SOCIAL CONTEXT INFLUENCES ON PAIN
Reviewing the growing literature on emotion and the experience of pain quickly underlines the intricacy of pain as a conscious experience. Not only can emotions triggered through different modalities lead to hyper- or hypoalgesia, but the emotional component of pain can also interact with the full range of attentional processes discussed earlier. Pain and emotion interaction at the experiential level is underpinned by interaction also at the cerebral level, as they share similar brain networks. The neural regions and networks specialized in the processing of emotional stimuli include notably the limbic and reward systems, which are also involved in cognitive-driven learning and conditioning. Although the joint contributions of these systems are often congruent, varying levels of attention and affect during pain may lead to opposing interactions and unexpected experiences.
As for pain, different brain networks with distinct functional roles tap into many of the same regions [28]. The motivational/affective/reappraisal component involving orbitofrontal, pgACC, and anterolateral prefrontal cortices is the most likely to interact with the limbic and the reward systems. Pinpointing how emotions can affect pain in different ways is a step toward acknowledging it as an experience that reaches far beyond nociception, and even far beyond immediate perceptions. Proper decoding and management of pain, an intruder in people’s consciousness, requires the appreciation of the full extent of this experience.
Similarly to the matricial versus vectorial distinction drawn about attention, affect can be divided into “mood” versus “discrete emotions.” Mood is the underlying affective state of an individual that emerges from a multitude of sources of information from past, present, and future events. Mood is related to traits and thus considered more stable, changing within a larger time window than discrete emotions, and typically assessed by subjective self-reported measures. Discrete emotions on the other hand are more rapidly changeable and often triggered by one specific source (although their expression will also be mood-dependent). Discrete emotions are thus more easily addressed in an experimental setting and will be reviewed here. In the clinic, however, consideration to both is warranted in the context of pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






