Fig. 1
LCN-2 protein levels in cortex (a) and hippocampus (b) after a sham operation (at day 1) or a TBI (at days 1, 3, and 7). Values are means ± SE, n = 3 for each group; * p < 0.05, # p < 0.01 vs. sham and contralateral tissues
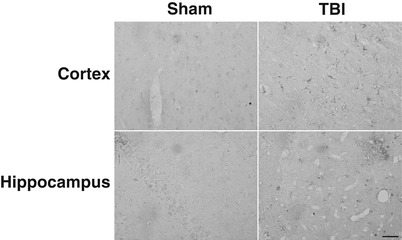
Fig. 2
LCN-2 immunoreactivity in cortex and hippocampus after a sham operation or a TBI at day 1. Scale bar = 50 μm
DFX treatment (100 mg/kg, intramuscularly, at 2 and 14 h after TBI) attenuated LCN-2 protein levels in both ipsilateral cortex (0.75 ± 0.24 vs. 1.23 ± 0.11 in vehicle, p < 0.05, Fig. 3a) and hippocampus (0.56 ± 0.14 vs. 0.93 ± 0.08 in vehicle, p < 0.01, Fig. 3b) at day 1 after TBI.
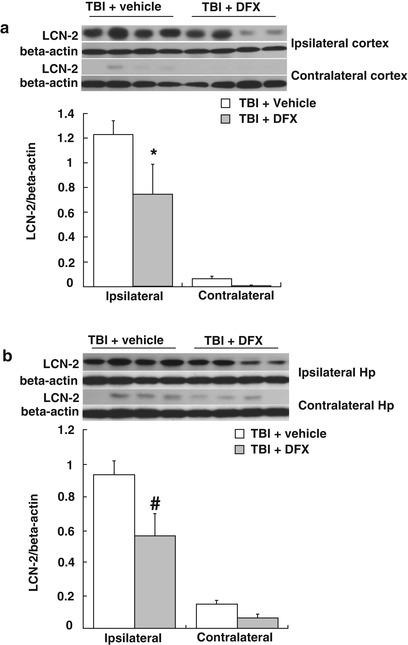

Fig. 3
LCN2 protein levels in the ipsi- and contralateral cortex (a) and hippocampus (b) at day 1 after TBI. Rats were treated with DFX (100 mg/kg) or vehicle. Values are means ± SE, n = 4 for each group; * p < 0.05, # p < 0.01 vs. vehicle group
Discussion
The major findings of the present study were (1) enhanced expression of LCN-2 in cortex and hippocampus after TBI and (2) systemic treatment with DFX attenuated the TBI-induced LCN-2 upregulation.
Lateral fluid percussion has been widely used to induce TBI since the 1980s. In the present study, TBI increased LCN-2 protein levels in the ipsilateral cortex and hippocampus with a peak at day 1. There was almost no LCN-2 expression in sham-operated groups or in the contralateral samples from the TBI groups. In our previous research in this TBI model, we have found hemorrhage in multiple locations, including subdural, subarachnoid, intraparenchymal, and intraventricular [18]. We have also found that LCN-2 is induced by an intracerebral injection of blood (100 μl), an ICH model, in rats [5]. In combination, these results suggest that the upregulation in LCN-2 after TBI is, at least in part, a response to the hemorrhage that occurs. It should be noted that LCN-2 upregulation in the ICH model was greater and longer lasting than found after TBI, and this may reflect the different size of hemorrhage in the two models.
After a cerebral hemorrhage, hemoglobin is released from lysed extravascular erythrocytes and degraded into heme, which is then further degraded into three components by heme oxygenase: ferric iron, carbon monoxide, and biliverdin. Our previous research reported iron levels in CSF increased on the first day, peaked on the third day, and remained high for at least 1 month after experimental ICH [14]. We have previously found that iron plays an important role in ICH-induced LCN-2 upregulation [5]. In the current study, the decrease in TBI-induced LCN-2 upregulation with DFX also suggests that iron is an important inducer of LCN-2 after TBI.
Evidence suggests that LCN-2 is involved in iron uptake into cells expressing the LCN-2 cell surface receptor, LCN2R [4]. Thus, the increase in LCN-2 after TBI may be involved in clearing hemorrhage-derived iron. However, whether LCN-2 has a beneficial or detrimental role in TBI has yet to be investigated. In spinal cord injury, LCN-2-deficient mice showed better neurological recovery [13]. Similarly, we have recently found that the absence of LCN-2 reduces SAH-induced white matter injury [6]. Further studies are needed to explore the effects of LCN-2 in TBI, whether it is detrimental, whether it may be involved in cellular iron overload, and whether it is involved in the post-TBI inflammatory response because LCN-2 is an acute phase protein.
Acknowledgment
This study was supported by the Joyce & Don Massey Family Foundation, and grants NSFC81301049 and NSFC81271374, 973 Program-2014CB541600, and an UMHS-PUHSC Joint Institute grant.









