Fig. 1
T2-weighted MRI images (a) and lateral ventricular volume measurements (b) from rats 24 h after injection of 50 μl of saline or thrombin (3 U) into the right lateral ventricle. Values are means ± SD, n = 6, # p < 0.01 vs saline group
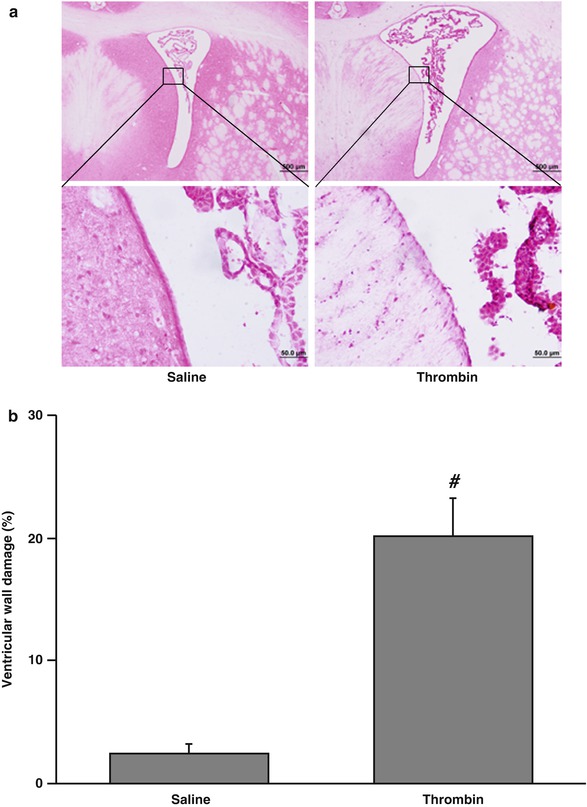
Fig. 2
The damaged ventricular wall in H&E-stained coronal sections (a) and the percentage of damage ventricular out of total ventricular wall (b) at 24 h after injection of 50 μl of saline or thrombin (3 U) into the right lateral ventricle. Values are means ± SD, n = 6, # p < 0.01 vs saline group
Systemic treatment with acetazolamide, a carbonic anhydrase inhibitor, 1 h after intraventricular injection of 3 U of thrombin resulted in less ventricular enlargement compared with vehicle treatment (16.1 ± 4.2 vs 29.5 ± 5.3 mm3 in the vehicle-treated group, p < 0.01) (Fig. 3).
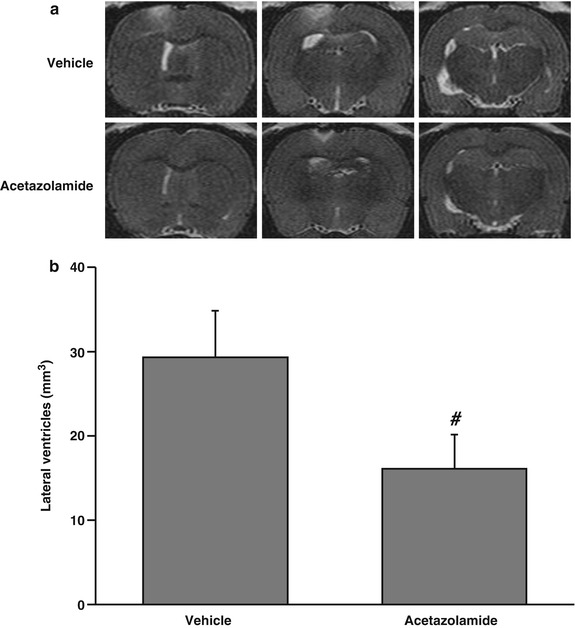

Fig. 3
T2-weighted MRI images (a) and lateral ventricular volumes (b) 24 h after injection of thrombin (3 U). Rats were treated systemically (IP) with vehicle or acetazolamide 1 h after the thrombin was injected. Values are means ± SD, n = 6, # p < 0.01 vs vehicle-treated group
Discussion
In this study, we found intraventricular injection of thrombin caused hydrocephalus and ventricular ependymal wall damage. Acetazolamide, given at 30 mg/kg IP 1 h after thrombin injection, attenuated thrombin-induced hydrocephalus.
Thrombin is a serine protease and an essential component in the coagulation cascade. Experimental investigations have indicated that thrombin formation plays a major role in ICH-induced injury [9, 11, 16]. Thrombin is responsible for early brain edema formation after ICH, and that edema results partly from a direct opening of the blood-brain barrier (BBB). We have demonstrated that intraventricular injection of thrombin can cause significant ventricular dilatation and periventricular parenchyma injury [6]. At present, the mechanisms associated with thrombin-induced ventricular dilatation are unknown. Ependymal injury such as reduced cilia and abnormal of organelle structure were observed in thrombin-injected rats [6]. Ependymal damage may lead to increased periventricular brain injury and to hydrocephalus [4, 14]. Activities of normal ependymal cilia are thought to direct cerebrospinal fluid (CSF) current toward the ventricular outlets. Previous study showed absent or functionally defective ependymal cell motile cilia may be a cause of hydrocephalus in a mouse model [1]. Therefore, ependymal damage and abnormal ependymal cilia may play a role in hydrocephalus induced by thrombin.
Acetazolamide, a carbonic anhydrase inhibitor, decreases CSF production in animal models [5, 10, 17]. Acetazolamide has a rapid onset of action. Treatment with 10 mg/kg of acetazolamide resulted in a significant decrease in CSF production and absorption within 3 h of ingestion in dogs [17]. Acetazolamide is also used in both children and adults to treat hydrocephalus and pseudotumor cerebri [6, 7]. We have shown that co-injection of acetazolamide reduced ICH-induced brain injury [7]. In the present study, acetazolamide treatment significantly reduced thrombin-induced hydrocephalus, most likely by reducing CSF production.
Conclusions
Intraventricular injection of thrombin caused ventricular wall damage and hydrocephalus in rats. Inhibition of carbonic anhydrase by acetazolamide effectively reduced thrombin-induced hydrocephalus. The results suggest that thrombin-induced hydrocephalus may result from reduced absorption and/or increased production of CSF.









