Fig. 3.1
Process of autophagy. A small volume of cytoplasm is enclosed by the isolation membrane, which results in the formation of an autophagosome. The autophagosome fuses with the lysosome where the cytoplasm is degraded. LC3 is bound to the autophagosomal membrane
Previous studies suggested that autophagy has a cytoprotective function against cell death [7, 8]. Autophagy contributed to cytoprotection in neurodegenerative disease and traumatic brain injury [9–13]. On the contrary, previous studies suggested that autophagy also contributes to the induction of cell death [8, 14, 15]. Autophagy can lead to nonapoptotic programmed cell death, which is called autophagic cell death [3, 16]. Activation of autophagy can induce cell death in a myocardial ischemia and reperfusion model [17]. In addition, autophagy can lead to autophagic cell death in cerebral ischemia and in a renal ischemia and reperfusion injury [18, 19].
Beclin 1, a Bcl-2-interacting protein, is a mammalian ortholog of yeast Atg6/Vps30 and it is known to be a promoter of autophagy [20]. Beclin 1 is a component of the class III phosphatidylinositol-3-kinase (PI3K) complex that works for the formation of autophagosomes [21]. The Atg8 protein, known as microtubule-associated protein 1 light chain 3 (LC3), is essential for autophagy [22]. LC3 is bound to autophagosomal membrane and thus is considered a specific marker protein to monitor autophagy [23] (Fig. 3.1). Autophagy may be dysregulated in several disorders, including metabolic diseases, neurodegenerative disorders, infectious diseases, and cancer. Pharmacological approaches to upregulate or inhibit this pathway are currently receiving considerable attention [24]. The mammalian target of rapamycin (mTOR) signaling pathway is known as a main molecular mechanism to regulate autophagic activity (Fig. 3.2). Recent studies revealed modulation of autophagy via mTOR signaling can be a therapeutic target for various diseases [24].
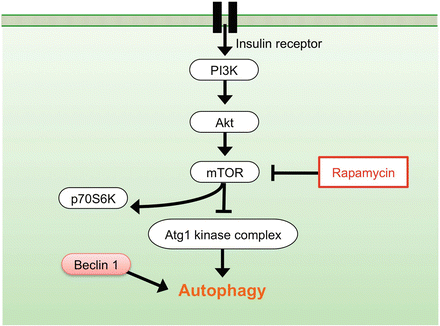

Fig. 3.2
Signaling pathway of autophagy regulation. mTOR pathway negatively regulates autophagy. Rapamycin, a specific inhibitor of mTOR, prevents phosphorylation of p70S6K and promotes autophagy. Beclin 1 protein promotes autophagy
We previously reported that autophagic activity was upregulated in damaged neural tissue after SCI [25–27]. Furthermore our study demonstrated that pharmacological enhancement of autophagy provided neuroprotective effect following SCI [28, 29]. Here, we summarize our previous studies and review the evidence in related articles regarding the role of autophagy in SCI.
3.2 Upregulation of Beclin 1 Expression After Spinal Cord Injury
3.2.1 Summary
Beclin 1, a Bcl-2-interacting protein, is known to be a promoter of autophagy. We previously investigated the alterations in the Beclin 1 protein expression and the involvement of autophagy after SCI using a spinal cord hemisection model in mice [25]. In our results of immunohistochemistry and Western blot analysis, the Beclin 1 expression significantly increased at the lesion site after hemisection. The Beclin 1 expression was observed in neurons, astrocytes, and oligodendrocytes. These results suggested that autophagy can be activated in the injured spinal cord.
3.2.2 Increased Expression of Beclin 1 After SCI
To investigate an alteration of the Beclin 1 expression in the spinal cord, immunohistochemical staining of Beclin 1 was performed at 4 and 24 h and 3, 7, and 21 days after hemisection. The cells expressing Beclin 1 were increased in the injured side after hemisection (Fig. 3.3a). The cells expressing Beclin 1 were observed in both the gray matter and white matter of the injured side. In counting Beclin 1-positive cells, the number of Beclin 1-positive cells on the injured side was significantly higher than those on the contralateral side at each time point. The increased expression of Beclin 1 started from 4 h, peaked at 3 days, and lasted for at least 21 days after hemisection. Western blot analysis confirmed that the level of Beclin 1 protein was significantly higher in the injured side than in the contralateral side.
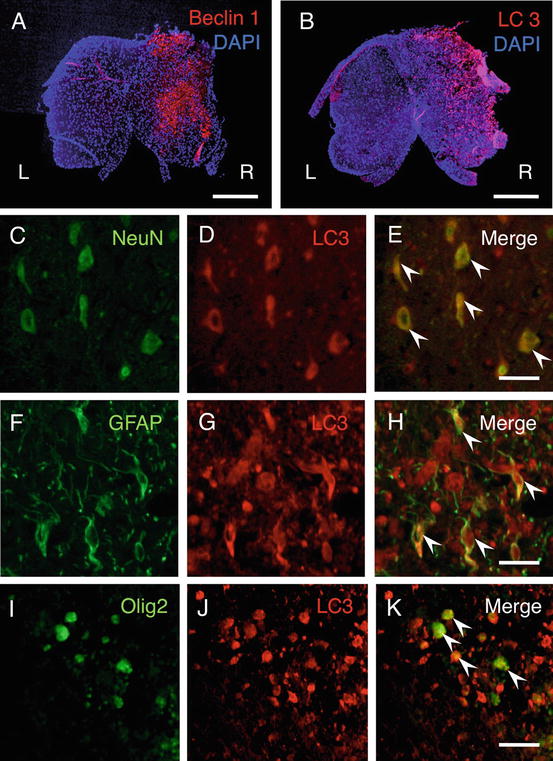

Fig. 3.3
Immunohistochemical staining of Beclin1 and LC3 in transverse sections at 3 days after hemisection. (a), (b) The cells expressing Beclin1 and LC3 were increased on the injured side (R) in comparison to the contralateral side (L). Scale bars = 500 μm. (c)–(k) In double staining of LC3 and cell type makers (green) on the injured side in transverse section at 3 days after hemisection, the LC3-positive cells were observed in the NeuN-, GFAP-, and Olig2-labeled cells (arrowheads in (e), (h), (k)). Scale bars = 50 μm
3.2.3 Beclin 1 Expression in Various Neural Cells After SCI
To investigate the Beclin 1 expression in a specific type of cells including neurons, astrocytes, and oligodendrocytes, the spinal cord sections at 3 days after hemisection were double stained for Beclin 1 and various cell type markers: NeuN for neurons, GFAP for astrocytes, and Olig2 for oligodendrocytes. In the double staining, the expression of Beclin 1 was observed in NeuN-, GFAP-, and Olig2-labeled cells. These results demonstrated the Beclin 1 expression to be observed in neurons, astrocytes, and oligodendrocytes.
3.2.4 Expression of Beclin 1 in Dying Cells
To detect Beclin 1 expression in dying cells, we performed double staining of Beclin 1 and TUNEL in the sections at 3 days after hemisection. The TUNEL-positive cells occasionally showed as Beclin 1 positive. Under higher magnification, most of the nuclei of the TUNEL-positive cells that did not show Beclin 1 positive were shrunken or fragmented, as is typical of apoptotic nuclei. On the other hand, most of the nuclei of the TUNEL-positive cells that were found to be Beclin 1 positive were round, as in autophagic cell death, and they had neither shrunken nor were fragmented.
3.3 Confirmation of Autophagy Induction After Spinal Cord Injury
3.3.1 Summary
To confirm induction of autophagy after SCI, we previously investigated expression of LC3, a characteristic marker of autophagy, in immunohistochemistry and Western blot using an SCI model in mice [26]. Electron microscopic analysis was also performed to examine the formation of autophagy in the injured spinal cord. Immunohistochemistry showed that the number of the LC3-positive cells significantly increased at the lesion site after hemisection. The LC3-positive cells were observed in neurons, astrocytes, and oligodendrocytes. Western blot analysis demonstrated that the level of LC3-II protein expression significantly increased in the injured spinal cord. Electron microscopy showed an increased formation of autophagic vacuoles in the damaged neural cells. This study confirmed both biochemically and anatomically that autophagy was clearly activated in the damaged neural tissue after SCI.
3.3.2 Upregulation of Autophagy Marker, LC3 in Injured Spinal Cord
Immunohistochemical analysis showed that cells expressing LC3 were increased on the injured side in comparison to the contralateral side after hemisection. The cells expressing LC3 were observed in both the gray matter and the white matter of the injured side (Fig. 3.3b). In higher magnification on the injured side, the cells expressing LC3 displayed bright, punctate LC3 dots in the cytoplasm, indicating formation of autophagic vacuoles. The number of LC3-positive cells on the injured side was significantly higher than those on the contralateral side at 3 days. The increase of the LC3-positive cells commenced at 4 h and lasted for at least 21 days. The maximum number of LC3-positive cells on the injured side was observed at 3 days, and it thereafter decreased at 7 days after hemisection. Western blot analysis confirmed the level of LC3-II protein was significantly higher in the injured side than in uninjured spinal cord.
3.3.3 LC3 Expression in Various Neural Cells
Double staining of LC3 and various cell type markers revealed that the LC3-positive cells were observed in NeuN-, GFAP-, and Olig2-labeled cells on the injured side after hemisection (Fig. 3.3c–k).
3.3.4 Electron Microscopic Analysis for Autophagy Formation
Electron microscopic analysis after the hemisection demonstrated that the formations of numerous autophagic vacuoles including autophagosome with double-membrane structures (Fig. 3.1) were observed in the damaged cells on the injured side. A higher magnification showed that the autophagosomes were containing membranous structures and parts of the cytoplasm.
3.3.5 Expression of LC3 in Dying Cells
To confirm autophagy induction in dying cells, we performed double staining of LC3 and TUNEL using the spinal cord section at 3 days after hemisection. The double staining showed that the TUNEL-positive cells were occasionally LC3 positive. Higher magnification revealed that the nuclei of the TUNEL-positive cells that were not LC3 positive were shrunken or fragmented, typical of apoptotic nuclei. On the contrary, the nuclei of the TUNEL-positive cells that were found to be LC3 positive were round, as in autophagic cell death.
3.4 Autophagy Modulation as a Potential Therapeutic Target for Spinal Cord Injury
3.4.1 Summary
The mTOR is a serine/threonine kinase that negatively regulates autophagy (Fig. 3.2). Rapamycin, an inhibitor of mTOR signaling, can promote autophagy and exert neuroprotective effects in several diseases of the central nervous system. We previously investigated whether administration of rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord contusion injury in mice [28]. Our results demonstrated that the administration of rapamycin at 4 h after injury significantly promoted autophagic activity in the injured spinal cord. In addition, the rapamycin treatment significantly reduced neural tissue damage and locomotor impairment after SCI. These results indicate that rapamycin promoted autophagy by inhibiting the mTOR signaling pathway and induced neuroprotective effect after SCI (Fig. 3.4).
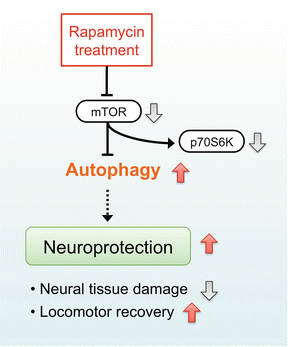

Fig. 3.4
Neuroprotective mechanism of rapamycin treatment in acute SCI. Rapamycin suppresses mTOR signaling pathway and promotes autophagy after SCI. The increased autophagic activity can produce neuroprotective effect and reduce neural tissue damage and locomotor impairment following SCI
3.4.2 Inhibition of mTOR Promotes Autophagy After Spinal Cord Injury
To examine the effectiveness of the rapamycin treatment on the mTOR signaling pathway, the phosphorylation of p70S6K was evaluated by Western blot analysis. In our results, the phosphorylated p70S6K protein was significantly decreased after administration of rapamycin, indicating rapamycin actually inhibited mTOR after SCI (Fig. 3.4). We also investigated the activation of autophagy after rapamycin treatment, immunohistochemical staining, and Western blot analysis of LC3 were performed. Immunostaining of LC3 showed the number of LC3-positive cells was significantly increased in the rapamycin-treated mice compared with the vehicle-treated mice. In the Western blot analysis, the expression of LC3-II protein was significantly increased in the rapamycin-treated mice compared with the vehicle-treated mice.
3.4.3 Inhibition of mTOR Produces Neuroprotective Effect in Injured Spinal Cord
To investigate neural cell loss after injury, the number of NeuN-positive cells was compared between the vehicle and the rapamycin-treated mice by immunohistochemical staining. In our results, the number of NeuN-positive cells in the rapamycin-treated mice was significantly higher than those in the vehicle-treated mice at 42 days. Additionally, to investigate the effect of rapamycin on cell death after SCI, we performed TUNEL staining and compared the number of TUNEL-positive cells between the vehicle- and the rapamycin-treated mice at 3 days after injury. The number of TUNEL-positive cells was significantly lower in the rapamycin-treated mice compared to the vehicle-treated mice. These results indicated that rapamycin treatment can produce neuroprotective effect to reduce neuronal loss and cell death following SCI.
3.4.4 Inhibition of mTOR Improves Locomotor Recovery After Spinal Cord Injury
To evaluate the effect of rapamycin treatment on locomotor recovery after SCI, Basso mouse scale (BMS) was measured for 6 weeks [30]. In our result, the rapamycin-treated mice had significantly higher BMS scores than the vehicle-treated mice from 3 to 6 weeks. This data supports the neuroprotection produced by mTOR inhibition can improve locomotor function after SCI.
3.5 Discussion
3.5.1 Upregulation of Autophagy in CNS Injury
Previous studies demonstrated the expression of Beclin 1 to increase at lesion sites after traumatic brain injury and cerebral ischemia [9, 18, 31]. In such diseases, expression of LC3 increases at the lesion sites [18, 32–35]. These reports suggest that the autophagic activity increases in response to the neural tissue damage of the brain. Our previous studies first reported the increased expression of Beclin 1 and LC3 in the damaged neural tissue after SCI [25, 26]. Using electron microscopy, we also confirmed that the formation of autophagic vacuoles also increased in the damaged cells of the injured spinal cord [26]. Therefore, autophagy was activated in the damaged neural tissue after SCI.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








