Pathogen
Overall mortality rate in uncomplicated
meningitis
Overall mortality rate in complicated
meningitis
Residual disability essentially encountered in infants
N. meningitidis
S. pneumoniae
H. influenzae
L. monocytogenes
6 %
13.5 %
0.9–9 %
15–30 %
13–32 %
24 %
–
–
9 % behavioral problem, 30 % neurologic deficits (seizures, hearing, language, mental, motor disorder)
31 % (deafness, later seizures)
25 % (deafness 6 %)
8.1.2 Brain Abscesses
Key Facts
Terminology and definitions – Brain abscesses.
Clinical features – Headache is the most common (70 %) symptom.
Blood – Positive culture in 10 % of cases.
CSF – Contraindicated.
Imaging: CT scan, MRI.
Prognosis
Principles of treatment – Antibiotics ad neurosurgery.
Disability – Mortality 100 % before the advent of antibiotics.
8.1.2.1 Terminology and Definitions
Brain abscess is an intracerebral collection of pus that often presents as a mass lesion causing focal neurological deficits related to the area involved. Primary source is often cryptogenic; 50 % caused by contiguous spread from the middle ear, paranasal sinuses, or dental foci, 25 % occurs after hematogenous spread from the lung (bronchiectasis, abscess) or heart (endocarditis, congenital defects), and 10 % are exogenous (fractures of the skull, intracranial surgery). Brain abscesses affect males more than females and involve 30–40-year-old patients most commonly.
8.1.2.2 Clinical Features
Headache is the most common (70 %) initial symptom of intracranial abscess. Other symptoms by frequency are drowsiness and confusion, focal or generalized seizures, focal motor, sensory, or speech disorders, nausea, and vomit. Fever (50 %) and leukocytosis are not always present, and the course of the disease can be indolent but sometimes rapidly life-threatening.
8.1.2.3 Diagnostic Markers
Diagnosis is based on a high index of clinical suspicion in patients with risk factors. Common microorganisms involved in brain abscesses are streptococci (70 % of cases), mostly anaerobic or microaerophilic. Infection is often polymicrobial (60 % of cases) with the involvement of other anaerobes and Enterobacteriaceae (20–50 % especially E. coli, Proteus). Staphylococci are also common (20 %), especially after neurosurgery. In immunocompromised hosts, Nocardia spp. should be considered.
Blood
– Positive cultures in 10 % of cases.
CSF
– Spinal tap is generally contraindicated, because intracranial pressure is elevated in many patients.
CT and MRI
– Are the most important diagnostic tools.
Chest X-Ray
– Is mandatory to rule out pneumonia, bronchiectasis, as original foci of infection.
8.1.2.4 Prognosis
The advent of antibiotics, new imaging, and stereotactic drainage has decreased the mortality near to zero [2].
Principles of Treatment
Empiric high-dose parenteral antimicrobials may be indicated after a complete diagnostic evaluation with intensive support and, occasionally, neurosurgical intervention. A third-generation cephalosporin plus metronidazole (eventually plus vancomycine or amikacin) is an adequate approach to the early stage of abscess formation (cerebritis). Abscess larger than 2.5 cm should be aspirated before starting an empirical therapy. Treatment must be targeted on the antimicrobial susceptibility tests. The duration of therapy depends on clinical and neuroimaging monitoring, and several weeks of treatment is advised. Intracranial pressure should be managed by the use of IV mannitol and dexamethasone 6–12 mg q6h.
Mortality and Disabilities
Prior to antibiotics and refinement of neurosurgical techniques, mortality approached 100 %. If the patient is comatose before appropriate treatment, mortality approaches 20–50 %; if the treatment starts when the patient is still alert, the range of mortality decreases to 5 and 10 %. Among the surviving patients, approximately 30 % complain of some neurological disorders, particularly of focal epilepsy.
8.1.3 Tuberculosis (TB)
Key Facts
Terminology and definitions – Mycobacterium tuberculosis.
Clinical features – TBM, tuberculoma myelitis. British Medical Council criteria for TBM.
Diagnosis
Skin: Mantoux.
Blood – IGRA test.
CSF – Lymphocytic pleocitosis, low glucose level (<5 mg/dL). Positive culture or PCR.
Imaging – CT scan/MRI, tuberculoma, hydrocephalus, meningitis, vasculitis.
Prognosis
Principles of treatment – Antituberculous drugs, surgery in case of hydrocephalus.
Disability – Mortality, 20–50 %. Residual deficits, 20–50 %.
8.1.3.1 Terminology and Definitions
TB is one of the most common infections by a single agent all over the world, and is second only to HIV. It is caused by Mycobacterium tuberculosis that is acquired through inhalation of airborne droplet nuclei with a possible secondary dissemination to other organs, such as CNS. CNS TB may affect immunocompetent hosts, but more commonly it involves immunocompromised ones, and in particular children (Fig. 8.1). CNS TB is five times more common in HIV-positive compared to HIV-negative patients.
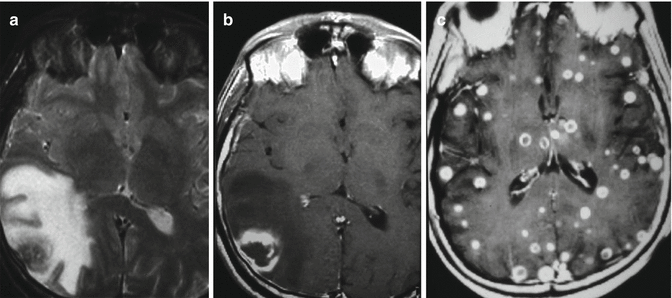

Fig. 8.1
Tuberculosis. Axial T2-weighted image (a) and axial T1-weighted image after contrast medium (b) in one patient with moderate immunodeficiency demonstrate a single right occipital–temporal granuloma characterized by a nodular necrosis associated with abundant perilesional edema and mass effect on the right ventricle. In another completely immunodeficient patient, a miliary tuberculosis is clearly evident in the axial T1-weighted image after contrast medium (c)
8.1.3.2 Clinical Features
TBM
Tuberculoma
Myelitis
The British Medical Council has established clinical criteria for the severity of TBM: stage I––fully conscious and no focal deficits; stage II––conscious but with inattention, confusion, lethargy, and focal neurological signs; stage III––stuporous or comatose, multiple cranial nerve palsies, or complete hemiparesis or paralysis.
8.1.3.3 Diagnosis
Skin
Intradermal reaction of Mantoux
Blood
IGRA test (low sensitivity)
CSF
Lymphocytic pleocitosis, low glucose level (<5 mg/dL). Culture for Mycobacterium tuberculosis, PCR (low sensitivity)
Cerebral CT scan/MRI
Tuberculoma, hydrocephalus, meningitis, vasculitis
8.1.3.4 Prognosis
Principles of Treatment
The standard approach to CNS tuberculosis includes an initial 2-month induction therapy regimen, including isoniazid, rifampin, pyrazinamide, and ethambutol, followed by 7–10 additional months of isoniazid and rifampin as maintenance therapy for an isolate that is sensitive to these agents. Treatment with corticosteroids is strongly recommended in CNS TB, in particular, because it reduces mortality from TBM. In case of severe hydrocephalus, surgical treatment is necessary to reduce the mass effect of tuberculomas and to drain brain abscesses.
Mortality and Disability
The clinical stage at diagnosis, the immunological status of the host, and antimicrobial resistance pattern of the strains are the main determinants of prognosis. Advanced stage, age, focal weakness, cranial nerve palsies, presence of any infarction other than a purely hemispheric infarction, and hydrocephalus are predictors of mortality at 3 months.
CNS tuberculoma is a benign condition with a good prognosis and effective therapy options. TB of the spine may evolve into paraplegia, spinal tumor syndrome, and kyphosis. Early diagnosis and prompt treatment can reverse paralysis and minimize the potential disability resulting from Pott’s paraplegia. When needed, a combination of conservative therapy and surgical decompression yields successful results in most patients with tuberculosis of the spine and who have neurological complications.
Mortality (prognosis quoad vitam) | Residual disability (prognosis quoad valetudinem) | |
|---|---|---|
TBM | HIV-negative 25 % HIV-positive 67 % | Frequency 20–50 % Hydrocephalus Cranial nerve palsies Ophthalmoplegia Seizures Psychiatric disorders Ataxia Hemiparesis Blindness Deafness |
8.1.4 CNS Syphilis
Key Facts
Terminology and definitions – Treponema pallidum.
Clinical features – Primary, secondary, and tertiary syphilis.
Diagnosis
Blood – treponemal and nontreponemal tests.
CSF – positive VDRL or RPR.
Prognosis
Principles of treatment – Penicillin G.
Disability – HIV is a predisposing factor to CNS syphilis. Tertiary syphilis may complicate with dementia paralytica, general paresis, and tabes dorsalis.
8.1.4.1 Terminology and Definitions
The diagnosis of neurosyphilis requires a CSF WBC count of 20 cells/μL or greater, or a reactive CSF Venereal Disease Research Laboratory (VDRL) test result. The burden from neurosyphilis is unknown, because national reporting of the disease is incomplete. Resurgence of early syphilis contemporaneously with the global epidemic of AIDS has renewed interest in syphilis pathogenesis and the host response.
8.1.4.2 Clinical Features
Syphilis is classified as primary (presence of chancre at the inoculation site), secondary (hematogenous dissemination), and tertiary (tabes dorsalis and general paresis, cardiovascular syphilis). Neurosyphilis can occur at any time in the course of syphilis.
Involvement of VIII cranial nerve can result in otosyphilis. Ocular involvement can occur (chorioretinitis, neuroretinitis, retinal vasculitis, and retinal detachment).
8.1.4.3 Diagnosis
Blood
– Treponemal (TPHA) and nontreponemal tests (VDRL, RPR).
CSF
– Pleocytosis (45 WBC per high power field), low glucose (2/3 serum glucose), and elevated protein (445 mg/dL). Positive VDRL or RPR
8.1.4.4 Prognosis
Principles of Treatment
The treatment recommendations for patients with neurosyphilis or ocular syphilis is aqueous crystalline penicillin G at 18–24 million units per day, either by continuous infusion or in divided doses, for 10–14-days.
Mortality and Disability
Immunosuppression due to human immunodeficiency virus (HIV) infection supports the development of neurosyphilis with parenchymal affection, predominantly if peripheral blood CD4-cell counts are below 350 cells/uL. The incidence of relapses or reinfections of patients after treatment of an early syphilis is believed to be more likely in HIV patients. Early and effective treatment may not only prevent further disease progression, but may also allow for a complete recovery.
Tertiary neurosyphilis may result in severe sequelae such as dementia paralytica or general paresis and tabes dorsalis not responsive to antibiotic treatment. Bilateral hearing loss may complicate ocular syphilis. Visual loss is common after chorioretinitis [5].
8.1.5 CNS Lyme Disease
Key Facts
Terminology and definitions: Borrelia burgdorferi.
Clinical features – Meningitis, encephalomyelitis, cranial neuropathies.
Diagnostic markers
Blood – Ab anti-Borrelia.
CSF – Ab anti-Borrelia.
Prognosis
Principles of treatment – Cephalosporins.
Disability – Limb paresis, chronic infection.
8.1.5.1 Terminology and Definitions
Lyme borreliosis is the most common human tick-borne disease in the northern hemisphere. The responsible pathogen is Borrelia burgdorferi sensu lato.
The incidence of Lyme borreliosis varies considerably from region to region.
8.1.5.2 Clinical Features
Nervous system infection with Borrelia burgdorferi frequently causes meningitis and rarely causes encephalomyelitis. Altered cognitive function also can occur in the absence of central nervous system infection. Cranial neuropathies are probably the most widely recognized neurologic presentations of Lyme disease. Approximately 80 % of these involve the facial nerve, causing unilateral or bilateral facial paralysis.
8.1.5.3 Diagnostic Markers
Blood
– Borrelia burgdorferi-specific IgM and/or IgG antibodies.
CSF
– Lymphocytic pleocytosis, detection of intrathecally produced, B. burgdorferi-specific antibodies. PCR and culture (very low sensitivity).
8.1.5.4 Prognosis
Principles of Treatment
Parenteral treatment regimens with third generation cephalosporins for 10–14 days. There is also a growing body of evidence that oral regimens, particularly with doxycycline can be highly effective.
Mortality and Disability
The outcome after antibiotic treatment is generally good. The pain, typical for Bannwarth’s syndrome, rapidly decreases under antibiotic therapy, and patients might be free of complaints even after one antibiotic dose. In the majority of cases, persisting objective symptoms after an adequate course of antibiotics are either due to irreversible damage (a limb paresis due to axonal loss) or might reflect a misdiagnosis in the majority of cases.
Because facial nerve palsy occurs frequently and is easily diagnosed, this group of patients has been the subject of several recent studies. If untreated, a significant number will develop persistent infection and late sequelae [6]. Some patients may develop a post-Lyme syndrome or chronic Lyme disease characterized by fatigue, malaise, and subjective perceptions of memory and cognitive impairment, but they usually have little compelling evidence of CNS infection or damage.
8.1.6 Infections of Ventriculoperitoneal Shunts
Key Facts
Terminology and definitions – Bacterial or fungal infections of the shunt.
Clinical features – Abdominal pain, fever (high in case of sepsis), meningeal signs, altered sensory and coma.
Diagnostic markers
Laboratory – Overturf’s diagnostic criteria for ventriculoperitoneal shunt infection.
–Blood – Positive culture.
–CSF – Positive culture.
Prognosis
Principles of treatment – Medical + surgical treatment (Staphylococcus aureus).
Disability – Fulminant in head trauma and postneurosurgical states. Increasing methicillin-resistant S. aureus (MRSA).
8.1.6.1 Terminology and Definitions
Ventriculoperitoneal shunting is a surgical procedure to treat excess cerebrospinal fluid (CSF) in the brain (hydrocephalus). Shunt surgery should be done as soon as hydrocephalus is diagnosed. Bacterial/fungal infection of the shunt, brain, or in the abdomen is one of the major risks of ventriculoperitoneal shunt placement (different studies report rates of CSF shunt infections ranging from 1.5 to 39 %).
8.1.6.2 Clinical Features
Infection from shunt may occur in case of a sepsis originating from other foci, or in case of abdominal infection causing peritonitis. Symptoms of infection manifest with abdominal pain, fever (high in case of sepsis), meningeal signs, altered sensory and coma.
8.1.6.3 Diagnostic Markers
Laboratory
Overturf’s Diagnostic Criteria for Ventriculoperitoneal Shunt Infection: [7].
Definite shunt infection: Compatible clinical signs and symptoms + Isolation of bacterial pathogen from device puncture, lumbar puncture, or other significant site (overlying shunt wound, cellulitis, or shunt tubing).
Probable shunt infection: Compatible signs and symptoms; CSF consistent with bacterial infection; Negative blood, CSF, and device cultures for bacteria.
8.1.6.4 Prognosis
Principles of Treatment
Medical + surgical treatment: IV or intrathecal antibiotics or antimycotic treatment based on the isolated microorganism (in empirical treatment, think about S. aureus, skin bacteria (Candida), and Gram-negative intestinal bacteria). Shunt removal and externalized ventricular drainage must be considered, because surgical treatment seems to be more efficacious than medical treatment, with a higher rate of initial cure, and lower morbidity and mortality rates.
Mortality and Disability
Morbidity and mortality depend on the age of the patient, the type of microorganism involved, and the underlying conditions. Fulminant course and fatalities are observed usually among adults with head trauma and postneurosurgical states; in contrast, pediatric cases following premature delivery involve both a more indolent course and a more favorable outcome [8]. Increasing MRSA strains affect treatment and prognosis of these kinds of infections. Prognosis of infections caused by Gram-negative bacteria is considered particularly poor: they are difficult to suspect because patients often appear relatively well at presentation, and are usually of mixed infections.
8.1.7 CNS Involvement in Endocarditis
Key Facts
Terminology and definitions – CNS complications of infective endocarditis.
Clinical features – Embolic occlusion and/or stroke. Mycotic aneurism.
Diagnostic markers
Blood – Positive culture.
Echocardiography – Findings of vegetating or ulcerative lesions suggestive for endocarditis.
Cerebral CT scan – Findings of vascular embolism.
Prognosis
Principles of treatment – IV antibiotic, delayed surgery.
Disability – Mortality 58–74 %.
8.1.7.1 Terminology and Definitions
The incidence of central nervous system (CNS) complications in infective endocarditis is approximately 30 %, and these manifestations are often the first sign of illness (47 % of the time in one series).
8.1.7.2 Clinical Features
Approximately 15–50 % of the CNS manifestations of infective endocarditis are due to embolic occlusion and/or stroke. Aneurysms of arteries may occur in around 5–10 %. A leak of a mycotic aneurysm or an underlying focal lesion can produce meningeal irritation and cause secondary aseptic meningitis. The overall prevalence of hemorrhage in CNS involvement of infective endocarditis is 3–7 %.
8.1.7.3 Diagnostic Markers
Blood
– Positive culture.
Echocardiography
– Findings of vegetating or ulcerative lesions suggestive for endocarditis.
Cerebral CT scan
– Findings of vascular embolism.
8.1.7.4 Prognosis
Principles of Treatment
Intravenous (IV) antibiotic treatment for 4–6 weeks (targeted on the isolated microorganism). Forty percent of patients with infective endocarditis will need cardiac surgery, primarily for valve repair or replacement. Timing of surgery in patients with infective endocarditis and embolic stroke remains controversial, but a report suggested that surgery can be performed relatively safely within 3 days of stroke, if heart failure is severe; otherwise, a delay of 2–4 weeks is preferable. In patients with associated hemorrhage, a delay of at least 4–6 weeks is preferred. Operative mortality is variable, but has been reported as 7.6 %. Anticoagulants are contraindicated because of the increased risk of CNS hemorrhage.
Mortality and Disability
Endocarditis can have profound and devastating neurological consequences [9]. In most cases, the neurological sequelae are present before the initiation of antimicrobial therapy (76 %). Studies have shown that the mortality of patients with bacterial endocarditis with CNS involvement is much greater (58–74 %) than that of patients who do not have CNS involvement (20–56 %). In one study, during the acute phase of infective endocarditis, 24 % of patients with neurological complications died, while only 10 % of those without neurological complications died. Among the patients with neurologic involvement, mortality was 25 % in those treated medically and 20 % in those treated surgically. In one series, 38 major embolic events occurred in 37 patients; 30 of these 37 patients died (81 % mortality).
8.2 CNS Fungal Infections
8.2.1 Aspergillus Species
Key Facts
Terminology and definitions – Aspergillus species
Clinical features – Cerebral abscesses, paranasal sinusitis, meningitis, meningoencephalitis, arachnoiditis, and ventriculitis. Mycotic aneurysms, cerebral infarcts, multifocal cerebritis.
Diagnostic markers
Lesion – Biopsy.
Blood – Aspergillus species: Galactomannan.
CSF – Galactomannan.
Prognosis
Principles of treatment – Voriconazole.
Disability – Mortality 50–80 %. Prognostic factor: immunosuppression.
8.2.1.1 Terminology and Definitions
The genus Aspergillus is an anamorphic member (asexual form) of the family Trichocomaceae. Aspergillus species are filamentous fungi spread worldwide, usually acquired by inhalation of Aspergillus conidia. CNS aspergillosis may result from: hematogenous dissemination, directed extension from the paranasal sinuses or eye, or direct inoculation. The most relevant risk factors for invasive aspergillosis include hematological disorders, prolonged and profound neutropenia (<100 neutrophils/ul), neoplastic diseases, solid organ transplantation, and allogenic hematopoietic stem cell transplantation. In this setting, the frequency of CNS infections ranges from 14 to 42 %.
8.2.1.2 Clinical Features
Cerebral abscesses, paranasal sinusitis, meningitis (usually associated with adjacent cerebral lesion), meningoencephalitis, arachnoiditis, and ventriculitis. Mycotic aneurysms, cerebral infarcts, multifocal cerebritis.
8.2.1.3 Diagnostic Markers
Lesion biopsy with direct examination and culture.
Blood
– Culture rarely positive. Presence of galactomannan (a cell-wall polysaccharide released by Aspergillus during growth).
CSF
– Culture is rarely positive. Inflammatory findings and hypoglycorrhachia. Presence of galactomannan in CSF.
8.2.1.4 Prognosis
Principles of Treatment
First choice: voriconazole. Second line therapy: itraconazole, posaconazole, liposomal amphotericin B. Therapeutic drug monitoring of voriconazole is needed to reduce drug toxicity. Radical surgical debridement for Aspergillus brain abscess.
Mortality and Disability
Immunosuppression and disseminated infection are risk factors of mortality. Outcomes of CNS aspergillosis appear to be improving after extensive use of voriconazole; complete and partial responses occurring in 35 % of patients, and 31 % surviving for a median observation time of 390 days. In case with space-occupying lesions, neurosurgical intervention is associated with improved survival. A CFR of 88.1 % is reported for disseminated and cerebral aspergillosis [11].
8.2.2 Candida Species
Key Facts
Terminology and definitions – Candida species.
Clinical features – Chronic meningitis, cerebritis, disseminated disease.
Diagnostic markers
Blood – Positive culture, Beta-D-glucan, mannan/antimannan.
CSF – positive culture, mannan.
Imaging – Microabscesses.
Prognosis
Principles of treatment – Liposomal Ampho B, fluconazole.
Disability: Mortality in ELBW = 30 %; 10–30 %, in general population.
8.2.2.1 Terminology and Definitions
Candida species are yeasts. C. albicans is the most common species responsible for candidal brain abscess (90 %). The organism is a normal commensal of humans. Usually, CNS candidiasis results from hematogenous dissemination, but Candida meningitis may result from infection from a ventricular shunt. Risk factors for neurocandidiasis are broad-spectrum antibiotic therapy, prematurity, parenteral nutrition, malignancy, treatment with corticosteroids, transplantation, indwelling devices, abdominal surgery, diabetes, dialysis, multiple sites colonization, and parenteral drug abuse.
8.2.2.2 Clinical Features
Chronic meningitis, diffuse cerebritis with multiple microabscesses (<3 mm), mycotic aneurysms: subarachnoid hemorrage and/or subdural empyema can appear. Approximately 50 % of patients with Candida meningitis have disseminated disease in other organs.
8.2.2.3 Diagnostic Markers
Blood
– Culture: positive in 50–60 % of cases of disseminated infection. Beta-D-glucan (a cell-wall component): high negative predictive value. Mannan antigen/antimannan antibody test.
CSF
– Culture: positive in 50 % of cases. Inflammatory findings with hypoglycorrhachia and lymphocyte pleocytosis. Positive CSF mannan.
MRI/TC
– Microabscesses in the joint between the gray and white matters; basal ganglia and the cerebellum.
8.2.2.4 Prognosis
Principles of Treatment
Liposomal amphotericin B with or without flucytosine is recommended for several initial weeks of treatment. Fluconazole is recommended as a step-down therapy. Removal of all infected devices is mandatory.
Mortality and Disability
Prognostic factors associated with a poor prognosis are (1) the delay to initiate antifungal therapy (more than 2 weeks after start of symptoms); (2) glycorrhachia < 35 mg/100 mL, (3) development of intracranial hypertension and focal neurological deficits.Extremely low birth weight infants (ELBW, birth weight <1000 g): death or neurodevelopmental impairment is observed for 73 % of ELBW infants who developed candidiasis [12]. Neurodevelopmental impairment occurs in nearly 53 % of children with Candida meningitis; also, cerebral palsy and blindness can occur (13.3 % and 6.7 % of patients, respectively). Vision impairment is observed in 46.7 % of patients. Also, hydrocephalus can develop.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





