Chapter 5
Basic Circadian Rhythms and Circadian Sleep Disorders
Circadian Rhythm Biology
Circadian rhythms are seen in all living creatures. The underlying mechanisms regulate nearly all physiological functions in the body, including the sleep-wake cycle. The two-process model postulates that sleep and wake are regulated by a homeostatic drive (process S) and a circadian system (process C) (Fig. 5.1).1 The homeostatic drive for sleep increases during wakefulness and dissipates during sleep, whereas the circadian process increases its alerting signal during the day to promote alertness and diminishes during the night to help promote sleep. The master clock of the circadian system is the suprachiasmatic nucleus (SCN), which is located in the anterior hypothalamus. The SCN regulates the circadian timing of most physiological and behavioral functions, including sleep and wake, core body temperature, hormones, and mood.2 The human circadian oscillation or period is approximately 24.2 hours and is regulated at the molecular level. The genetic basis of the circadian system has recently been elucidated. The involved genes are highly conserved and use an autoregulatory feedback loop with translocation of protein products from the nucleus to the cytoplasm and back to the nucleus. The rate of transcription determines the period of the circadian rhythm.3
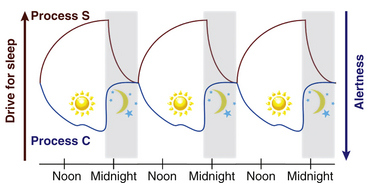
FIGURE 5.1 The two-process model of the sleep-wake cycle. Process S (red line) represents the homeostatic need for sleep that increases during wake and dissipates during sleep. Process C (circadian drive; blue line) increases alerting signals during the day to promote wake and decreases shortly before sleep onset.
Light is the strongest zeitgeber (German for time giver) that regulates circadian timing. The SCN receives input from light via specialized retinal photoreceptors containing melanopsin. Through this pathway, light input to the SCN inhibits pineal secretion of melatonin (Fig. 5.2); melatonin, once secreted, can also act as a zeitgeber and alter the phase of circadian rhythms.
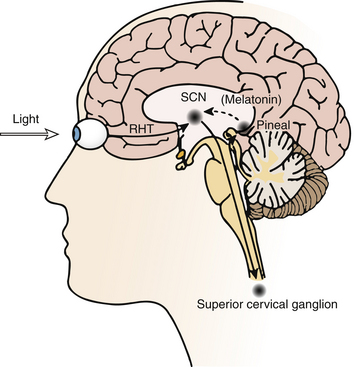
FIGURE 5.2 Schematic diagram of the neural pathways for light input to the circadian system. Light information from the melanopsin-containing retinal ganglion cells travels to the suprachiasmatic nucleus (SCN) via the retinohypothalamic tract (RHT). The SCN regulates the timing of nocturnal melatonin secretion from the pineal gland via the superior cervical ganglion. Exposure to bright light during the biological night suppresses melatonin production. (Modified with permission from Reid KJ, Zee PC. Circadian rhythm disorders. Semin Neurol. 2009;29[4]:393-405.)
Circadian timing can be modulated by controlling exposure to these zeitgebers, which can advance or delay the timing of circadian rhythms as predicted by the phase response curve (PRC) (Fig. 5.3).4,5 The direction of phase shift is dependent on the timing of zeitgeber exposure in relation to circadian phase. Biomarkers of circadian phase include the onset of melatonin secretion under dim light conditions (dim-light melatonin onset; DLMO) and core body temperature (CBT). Melatonin secretion begins about 2 hours before habitual bedtime, as the CBT begins to fall. Plasma melatonin levels peak during the CBT nadir and then fall as the CBT rises.6 For example, light exposure before the CBT nadir delays the timing of circadian rhythms, whereas exposure after the CBT nadir results in an advance. In contrast, exposure to melatonin before the CBT nadir advances and after the CBT nadir delays circadian rhythms.
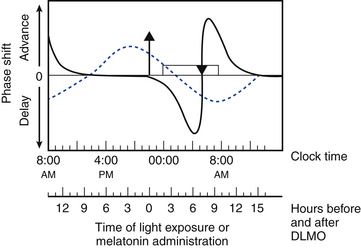
FIGURE 5.3 Schematic representation of the phase response curves (PRCs) to light (solid line) and melatonin (dotted line). The upper x-axis shows clock time, and the lower x-axis shows the time relative to dim-light melatonin onset (DLMO), a circadian phase marker. The y-axis represents the degree of phase advance or delay. Typical clock times are shown for the DLMO (10 PM), core body temperature (CBT) nadir (5 AM), and sleep times (12 AM to 8 AM). DLMO typically occurs 2 hours before biological sleep-onset time. The timings of these markers for individuals with delayed sleep phase disorder or advanced sleep phase disorder should be adjusted accordingly. The light PRC shows that light exposure between the DLMO and CBT nadir causes phase delay; after the CBT nadir, light exposure results in phase advance. The magnitude of phase advance or delay is greatest close to the CBT nadir. The melatonin PRC (generated using melatonin 0.5 mg) shows that melatonin exposure results in phase advance before 2 AM and phase delay after 2 AM. The greatest phase delay occurs 3 hours before DLMO, or 5 hours before sleep onset. Rectangle, Sleep period; triangle, CBT nadir; upward arrow, DLMO. (Modified with permission from Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6[5]:407-420.)
Circadian Rhythm Sleep Disorders
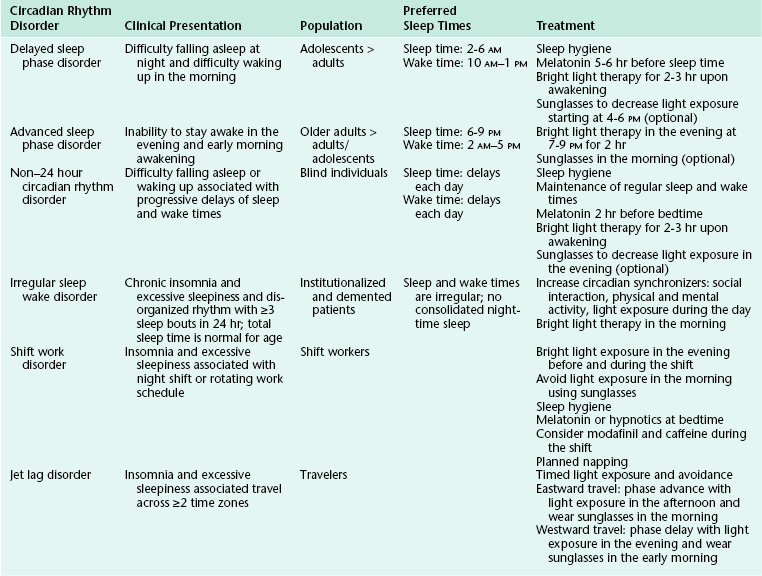
Circadian rhythm sleep disorders result from dysfunction of the circadian timing system or from misalignment between biological circadian phase and behavioral sleep and wake rhythms. Disruption of circadian timing results in insomnia, excessive daytime sleepiness, and impaired performance. Symptoms may cause disruption in social, occupational, and other areas of function. A summary of these disorders is shown in Figure 5.4 and Table 5.1.
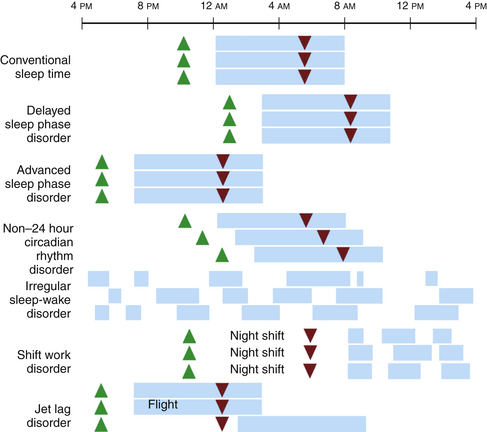
FIGURE 5.4 A schematic representation of circadian rhythm sleep disorders. Blue rectangles denote sleep time. Green triangles denote the dim-light melatonin onset, which occurs 2 hours before biological sleep-onset time. Red triangles denote the core body temperature nadir. Individuals with delayed sleep phase disorder have stable circadian rhythms that are delayed relative to conventional times, resulting in difficulty falling asleep and difficulty waking up in the morning. Those with advanced sleep phase disorder have stable endogenous rhythms that are early relative to conventional times, resulting in early evening sleepiness and early morning awakening. In non–24 hour circadian rhythm disorder, the rest/activity gradually delays each day, leading to difficulty falling asleep and daytime sleepiness, depending on when the circadian time for sleep falls on a particular day. Irregular sleep-wake rhythm disorder is characterized by a lack of consolidated sleep and at least three bouts of sleep each day; 24-hour total sleep time is normal for age. In shift work disorder, people working during the night shift work during the circadian time for sleep, which results in sleepiness and impaired performance. They sleep when circadian alerting signals are high, leading to disrupted and shorter sleep. Travelers flying two or more time zones may experience jet lag disorder. In the example given, westward travel results in the traveler’s circadian phase being advanced relative to the conventional sleep time in the destination, resulting in afternoon and evening sleepiness and early morning awakening.
Delayed Sleep Phase Disorder
Delayed sleep phase disorder (DSPD) is one of the most common circadian rhythm sleep disorders in which the circadian timing is delayed relative to the required sleep and wake times. This disorder is more common in adolescents and young adults. Symptoms include difficulty falling asleep at the desired bedtime and difficulty waking up at the desired wake time. When the person is allowed to sleep at the biologically correct time, sleep duration and architecture are typically normal. Sleep logs and actigraphy for 7 days and DLMO measurement can be helpful for distinguishing DSPD from other causes of insomnia or excessive daytime sleepiness (Fig. 5.5).7,8
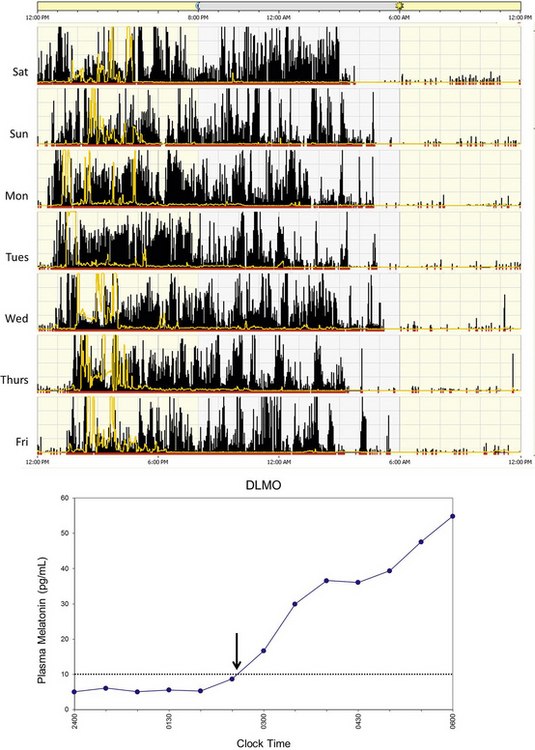
FIGURE 5.5 Above, An example of rest-activity cycle from a patient with delayed sleep phase disorder using wrist actigraphy monitoring. The black bars indicate activity level, and yellow lines indicate ambient light exposure at the nondominant wrist. This individual had a typical bedtime of 3 to 5 AM and wake time of 12 to 2 PM. Below, An example of plasma dim-light melatonin onset (DLMO) of the same individual. In plasma, DLMO is defined as the time at which the melatonin level reaches 10 pg/mL. The DLMO typically occurs about 2 hours before sleep onset. In this individual the DLMO was at about 2:30 AM (arrow) and sleep time was at 4:30 AM.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree