Marker profile
Cell type
Brain tissue
Reference
Toll-like receptors 2 and 4
Adult NSC/NPC
Mouse dentate gyrus
Rolls et al. 2007
CD133, Musashi-1, Sox2, melk, PSP, bmi-1, and nestin
Adult NSC
Human brain
Hemmati et al. 2003
GD3
Adult NSC
Mouse striata and subventricular zone
Nakatani et al. 2010
HRD1+/Nestin+/GFAP+
Adult NSC
Mouse subventricular zone
Kawada et al. 2011
EGF receptor
Adult NSC/NPC
Mouse subventricular zone
Doetsch et al. 2002
Ki-67
Adult NSC/NPC
Pig subventricular zone
Liard et al. 2009
Querkopf
Adult NSC
Mouse subventricular zone
Sheikh et al. 2012
Vimentin+, Nestin+, Sox2+
Radial glial cells
Rat germinal zone E 16.5
Li et al. 2011
CXCR4
Radial glial cells
Embryonic spinal cord
Mithal et al. 2013
CD133+/CD15+
Embryonic NSC
Human fetal brain E50–55
Sun et al. 2009
E-PHA binding N-glycans
Embryonic NSC
Mouse fetal brain E12, E14, E16
Hamanoue et al. 2009
Musashi1 and Musashi2
Embryonic NSC
Mouse embryonic forebrain
Sakakibara et al. 2002
Syndecan-1, Integrin-b1, and Notch-1
Embryonic NSC
Mouse embryonic forebrain E14.5
Nagato et al. 2005
Nestin, GFAP
Embryonic NSC and NPC
Rat embryonic forebrain E14.5
Martins et al. 2008
Toll-like receptor 3
Embryonic NSC
Mouse embryonic forebrain E14
Yaddanapudi et al. 2011
Pax-6
Embryonic NSC and NPC
Mouse embryonic forebrain
Estivill-Torrus et al. 2002
Sox 1, Sox 2, CD133
Embryonic and adult NSC
Mouse embryonic forebrain E12.5 Mouse subventricular zone
Corti et al. 2007
1.2 Neural Stem Cells in Developing and Adult Brain
CNS development starts with neural tube formation from the ectoderm. During early stage of CNS development , neuroepithelial progenitor cells through symmetric divisions expand the neuroepithelium. Later these cells by asymmetric division give rise to a stem cell and a more differentiated neural progenitor cell (NPC) cell characterizing neurogenesis initiation (Fig. 1.1). Overall, temporally and spatially distinct multipotent cell populations are widely present in the CNS tissue and respond to specific microenvironmental cues regulating proliferation and differentiation of these cells and being crucial for acquisition of the final cell phenotype and regionalization of the brain.
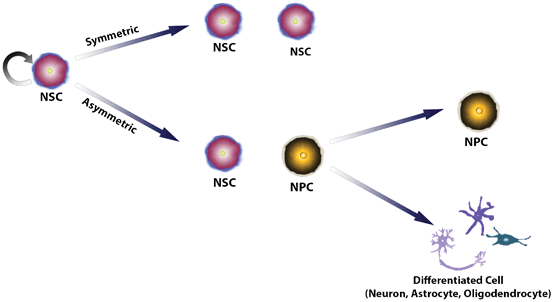
Fig. 1.1
Scheme of cell division promoting self-renewal and differentiation of neural stem cells
In the mouse, initial primitive NSC from E5.5 to 8.5 are responsive to leukemia-inhibitory factor (LIF) or fibroblast growth factor (FGF). Then, NSC expand in the ventricular zone and respond to epidermal growth factor (EGF) and FGF on E13. Peaks of neurogenesis occur on E14.5 involving Notch signaling and platelet-derived growth factor (PDGF) receptor activation. With the progression of development, the number of NSC begins to decrease and represents only a small percentage of cells present in specific regions of the brain (reviewed by Ramasamy et al. 2013). In addition to the above-mentioned cues, one of the crucial factors for forebrain patterning is the bone-morphogenetic protein (BMP) , which in conjunction with Wnt, FGF, and Sonic hedgehog (Shh) signaling determines dorsal-ventral forebrain patterning (Harrison-Uy and Pleasure 2012). Inibition of BMP-induced signaling is necessary for neural induction (Pera et al. 2014). Some cells from the neuroepithelium, known as radial glial cells, become bipolar and extend processes, which provide support for the migration of newborn neurons . These radial glial cells, regarded also as stem cells in neocortical development, are capable of self-renewal and originate differentiated neural cells, including neurons (Pinto and Gotz 2007; Gan et al. 2014). These cells expressed markers such as CXCR4, Vimentin, Nestin, and Sox2, characteristic for the NSC phenotype (Li et al. 2011) .
In adult brain, NSC are found in the subgranular zone (SGZ) of the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (Yamashima et al. 2007). Some molecules, such as BMP, Notch, and Shh, known to be involved in embryonic development of the CNS, are also presents in neurogenic regions of adult brain, and are also important for adult neurogenesis . Adult NSC respond to stimuli from the microenvironment and can proliferate, migrate to other regions of the brain and give rise to different neural phenotypes and functionally integrate into local circuits (Van Praag et al. 2002; Carleton et al. 2003; Gage and Temple 2013; Sequerra et al. 2013). Although NSC derived from embryonic and adult CNS are considered as multipotent, it is believed that there are temporal limitations for the potency of adult stem cells (Frantz and McConnell 1996; Nguyen et al. 2013) .
The establishment of protocols for isolation and culture of NSC has been of great value, both for basic research in order to understand mechanisms of neural development and adult neurogenesis as well as therapeutic applications. NSC can be isolated from the adult brain tissue only in small amounts, making difficult their use in cell replacement therapy. A therapeutic concept could be to isolate these cells during biopsy and then replicate them in vitro to increase the number of cells prior to using them for transplantation purposes .
Alternatively, NSC obtained from fetal CNS or can be derived from other stem cell types, such as embryonic stem cells (Garzón-Muvdi and Quiñones-Hinojosa 2009). More recently, NSC are being obtained by reprogramming mature somatic cells into induced pluripotent stem cells or direct-induced NSC (Li et al. 2013). We will focus on embryonic/fetal and adult brain NSC .
1.3 Neural Stem Cells in Culture
NSC usually are isolated and expanded in culture using two different strategies: (1) Neurospheres, which are three-dimensional structures maintained in suspension originated from a single cell by asymmetric division and constituted by NSC and NPC at different stages of differentiation (Suslov et al. 2000) . (2) Monolayer culture using adhesive substrate such as poly-lysine, poly-ornithine, laminin and fibronectin. The proliferative capacity of NSC is very similar using both methods. However, the neurosphere culture reveals a higher potential for generating neurons compared to NSC cultured in monolayers (Meyer et al. 2012). In fact, neurospheres and other cultures of neuroepithelial cells contain low numbers of NSC and a much higher percentage of NPC with restricted differentiation potential.
The neural tissue of the developing or adult CNS tissue is dissociated in vitro providing single cells that are cultured as neurospheres or adherent monolayer at low density in growth medium containing hormonal supplements (B27 and N2) and the growth factors EGF and basic FGF (Kornblum 2007; Negraes et al. 2012). The supplemented medium with growth factors maintains the ability of neurospheres to proliferate and prevents spontaneous differentiation of the cells . The removal of growth factors induces the differentiation of these cells into neurons, astrocytes and oligodendrocytes (Reynolds and Weiss 1996; Meyer et al. 2012). Using embryonic telencephalon rat E14.5 neurospheres, following 7 days of differentiation induction , a radial pattern of cell migration is observed together with an increase in neuron-specific β-3 Tubulin and glial specific GFAP (glial fibrillary acidic protein) expression from 25 to 75 % and 20 to 35 % of the cell population, respectively, while the percentage of Nestin-positive cells declines following induction of NSC to differentiation and the occurrence of oligodendrocytes remains below 10 % of the cell population. This cell system provides simplified conditions for studying the effects of extrinsic and intrinsic parameters on embryonic cortex development (Trujillo et al. 2012). Induction of differentiation results yet in a further increase of the number of neurons in the differentiated neurosphere population (Schwindt et al. 2011), while astroglial cell populations are enriched in long term cultures of neurospheres (Chiang et al. 1996) .
1.4 Proliferation, Migration and Differentiation
Neurogenesis is a highly regulated process in both development and the adult brain, where bioactive compounds are produced and released by the proper stem cells of the neurogenic niche. These extracellular messengers act together with intrinsic cues on proliferation , migration and differentiation of newborn cells . Growth factors , hormones, extracellular matrix molecules, neurotransmitters, cytokines, small non-coding RNAs have been related with regulation of NSC mobilization (Trujillo et al. 2009; Trujillo et al. 2012; Schouten et al. 2012; Oliveira et al. 2013; Bellenchi et al. 2013).
Neurogenesis is also modulated by the activity of ion channels. The concept of depolarization-driven progress of neural differentiation and phenotype determination goes back to the fundamental studies of Nicholas Spitzer (Ben-Ari and Spitzer 2010). Different checkpoints need to be passed for putting control over proliferation , migration and neurotransmitter and receptor specification. In view that, acetylcholine receptor expression patterns guide the progress of neurogenesis (reviewed by Trujillo et al. 2009; Berg et al. 2013). Activation of α7 nicotinic acetylcholine receptors inhibits proliferation and promotes neuronal differentiation of NSC (Narla et al. 2013). Moreover, the selective lesion of cholinergic input decreases neurogenesis in the dentate gyrus (Mohapel et al. 2005). The activation of muscarinic M1 receptors in the hippocampus induces neurogenesis (Ma et al. 2004; Van Kampen and Eckman 2010), as described as well for M2 receptors in the ventricular zone of the embryonic rat cortex (Resende and Adhikari 2009).
Dopaminergic receptors are involved in regulation of proliferation and differentiation of NPC in the SVZ (O’Keeffe et al. 2009). Stimulation of NPC proliferation by dopamine can be mediated by EGF (O’Keeffe et al. 2009). Moreover activation of dopamine receptors in neurospheres from the SVZ stimulate brain-derived neurotrophic factor (BDNF) release, increase cell proliferation and the number of differentiating cells (Merlo et al. 2011).
Involvement of glutamate receptors in proliferation of precursor cells depends on the expressed subtype and involves expression of neurotrophins (Mackowiak 2002). It has been shown that inhibited NMDA-glutamate receptor activity results in increased neurogenesis (Nacher et al. 2003), while activation of the metabotropic mGluR5 receptor augments NPC proliferation (Di Giorgi-Gerevini et al. 2005). SVZ progenitor activities depend on reactive astrocytes, which release glutamate and ATP resulting in activation of glutamate and purinergic receptors (Guo et al. 2013; Boccazzi et al. 2014).
Furthermore, some studies suggest that trophic factors such as BDNF, glial cell line-derived neurotrophic factor (GDNF) and vascular endothelial growth factor (VEGF) act in conjunction with neurotransmitter systems on neurogenesis (Berg et al. 2013). BDNF promotes differentiation and maturation of progenitor cells by enhancing GABA release in the SGZ (Waterhouse et al. 2012). This neurotrophic factor promotes neuronal migration via p75 neurotrophin receptor (NTR) activation in the adult SVZ (Snapyan et al. 2009). It has been suggested that adult neurogenesis augments by environmental enrichment and voluntary physical exercise. Bekinschtein et al. (2011) suggest that this effect results from BDNF released by newborn neurons.
Platelet-derived growth factor-BB (PDGF-BB) leads to increased neuronal differentiation and maintains the proliferation and migration of NPC . Such actions were observed in the embryonic ventricular zone and in post-natal brain development (Smits et al. 1991; Sasahara et al. 1992). Promotion of neurogenesis by PDGF-BB was also observed in an animal model of impaired hippocampal neurogenic proliferation inflicted by HIV Tat & cocaine (Yao et al. 2012).
Bradykinin, a biologically active peptide classically involved in inflammation and blood pressure regulation, performs its functions by binding to G protein coupled kinin-B2 receptors. Activation of B2 receptors results in proliferation block and induction of NPC migration and neurogenesis. Inhibition experiments in the presence of the selective B2 receptor blocker HOE-140 showed an increase in gliogenesis over neurogenesis as well as down-regulation of muscarinic and purinergic receptor activity and expression, indicating that bradykinin participated in neural fate determination (Trujillo et al. 2012).
Metaloproteinases (MMP) have been attributed with functions in proliferation and differentiation of NPC in the embryonic development of the mouse brain (Tonti et al. 2009). As extensively reviewed by Tonti and co-workers, their participation is suggested in embryonic and postnatal developmental neurogenesis by degrading extracellular matrix and allowing for neural migration . While MMP-9 is highly expressed in early developmental stages together with migrating external granular cell precursors, MMP-2 expression is observed during postnatal development. MMP-2 also seems to be involved in synaptic plasticity, as increasing activity of the enzyme was related to the recovery of plasticity along formation of cortex projection to the hippocampal dentate gyrus (Dzwonek et al. 2004).
Previous reports have shown the involvement of immune mediators in the control of neurogenesis in the adult brain (Gonzalez-Perez et al. 2010; 2012). Some studies suggest that inflammatory cytokines promote stimulating effects on neurogenesis especially in brain damage situations (McPherson et al. 2011). The brain is considered an immune-privileged environment due to the selectivity of blood-brain barrier, which limits the entrance of molecules and cells of the peripheral immune system and the presence of microglia responsible for the identification of signs of injury and inflammation . However, in the healthy brain, both during development and in the adult brain, the microglia is involved in phagocytosis of apoptotic cells . It is believed that microglia releases cytokines and free radicals acting on surrounding cells, thereby in dependence on the environmental situation promoting survival or death of such cells (Marín-Teva et al. 2011). Microglia is present in neurogenic niches and suggested to regulate neurogenesis by interacting with NSC.
Several cells in the brain including microglia, astrocytes and neurons produce and release cytokines, chemokines and express receptors for these factors, providing autocrine and paracrine loops for modulation of migration, fate choice and viability of NPC of the SVZ NPC (Gordon et al. 2012). Cytokines may have pro- or anti-inflammatory actions. While interferon-γ (IFN- γ), interleukin (IL)-1β, 6 and 18 and tumor necrosis factor (TNF)- α promote inflammatory reactions, insulin-like growth factor (IGF)-1 and IL-15 have anti-inflammatory properties, and IL-4 exerts opposing effects under different cellular conditions. However, their properties in augmenting or reducing inflammatory events cannot be directly linked to their inflammation-promoting or -inhibiting capabilities .
The pro-inflammatory cytokine TNFα leads to increased proliferation and differentiation into astrocytes and decreased the number of neurons. IFN-γ augments differentiation and neurite outgrowth in NSC, while it is also involved in reduction of proliferation and survival of NPC. On the other side, IL-1β inhibits proliferation and differentiation of NSC , such as has been observed for IL-6.
The anti-inflammatory cytokine IGF1, produced by activated microglia, is related to the promotion of neurogenesis. IL-4 leads differentiation to oligodendrocytes. In addition, NPC decreases proliferation and promotes the migration of these cells. IL-15 inhibits differentiation and self-renewal capacity of NSC. IL-18 produced by astrocytes and microglia acts by decreasing differentiation and promoting neuronal apoptosis. Leukemia inhibiting factor (LIF) and ciliary neurotrophic factor (CNTF), known to maintain pluripotency in embryonic stem cells during replication (Wolf et al. 1994), are also important for the self-renewal capacity of NSC (Deverman and Patterson 2009; Gonzalez-Perez et al. 2010, 2012; Yoneyama et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








