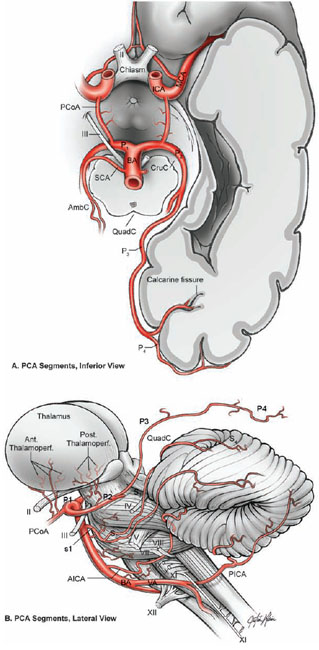
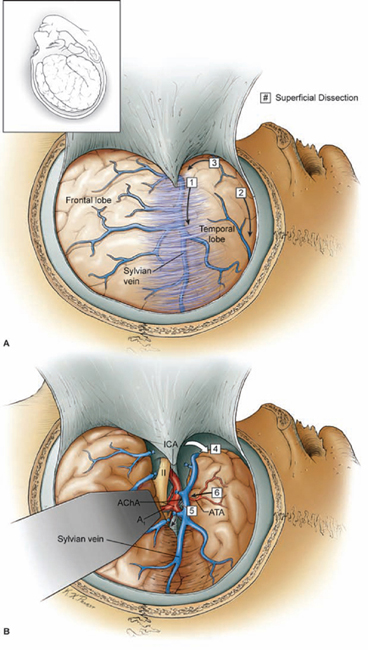
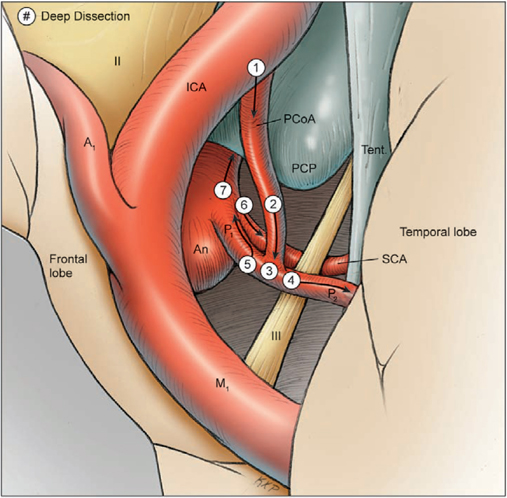
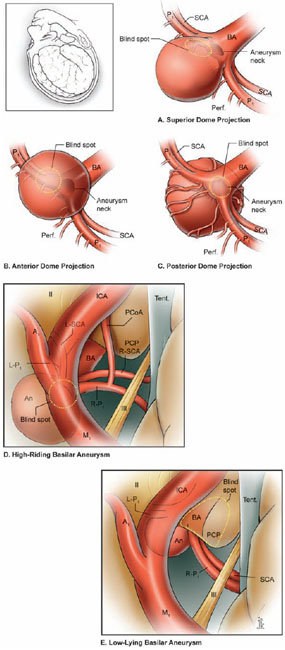
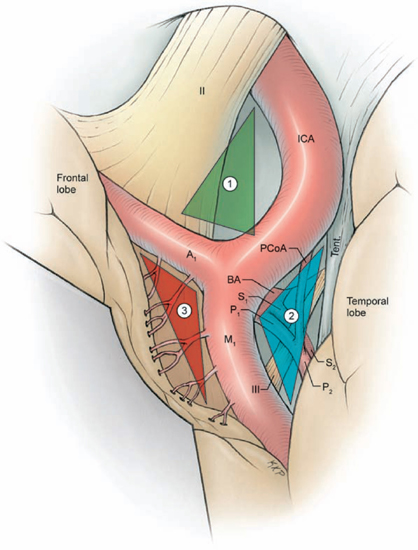
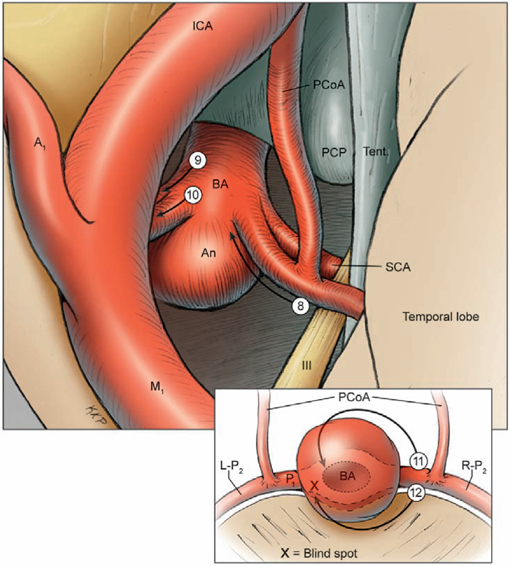
19 Basilar Artery Bifurcation Aneurysms The basilar artery terminates in a quadrifurcation from which two posterior cerebral arteries (PCAs) and two superior cerebellar arteries (SCAs) originate (Fig. 19.1). The P1 segment of the PCA begins at the basilar bifurcation and ends at the junction with the posterior communicating artery (PCoA). This precommunicating segment is typically larger in caliber than the PCoA, but may be smaller in patients with fetal anatomy. The P2 segment or post-communicating segment begins at the junction with the PCoA and ends lateral to the posterior margin of the midbrain. It is sometimes divided into anterior and posterior halves. The P2A segment or crural segment courses around the cerebral peduncle to its lateral edge through the crural cistern, and the P2P segment or ambient segment courses around the lateral midbrain through the ambient cistern. The P3 segment or quadrigeminal segment begins lateral to the posterior margin of the midbrain, climbs over the free edge of the tentorium, courses through the quadrigeminal cistern, and ends at the anterior limit of the calcarine fissure. The PCA typically branches along this segment into its major terminal arteries: the calcarine and parieto-occipital arteries. The P4 segment or calcarine segment begins at the anterior limit of the calcarine fissure and ends at the cortical surface of the occipital lobe. The nomenclature of numbered arterial segments ends in the supratentorial compartment, but the infratentorial arteries also have well-defined segmental anatomy. The SCA is divided into four segments: anterior pontomesencephalic, lateral pontomesencephalic, cerebellomesencephalic, and cortical. The anterior pontomesencephalic segment begins at the SCA origin, courses underneath the CN3, and ends at the anterolateral brainstem, medial to the tentorial edge. The lateral pontomesencephalic segment begins at the anterolateral brainstem, dips caudally to the trigeminal root, and terminates at the entrance to the cerebellomesencephalic fissure. The cerebellomesencephalic segment courses posteriorly within this fissure, following the trochlear nerve and superior cerebellar peduncle. The cortical segment begins as distal branches exit the cerebellomesencephalic fissure and supply the cerebellum’s tentorial surface. Variations in SCA anatomy include a common origin with the PCA or an origin from the P1 segment. Dissection of basilar bifurcation aneurysms does not depend on the distal anatomy of the PCA and SCA, only the P1 segments bilaterally, anterior pontomesencephalic SCA segments bilaterally, basilar trunk, and thalamoperforating arteries. The posterior thalamoperforators arise from the P1 segments, with the majority arising from the middle third as individual branches or branching trunks that can be bilateral and symmetric, bilateral and asymmetric, or unilateral with bilateral territory (artery of Percheron) (Fig. 19.2). The posterior thalamoperforators supply the anterior and part of the posterior thalamus, hypothalamus, subthalamus, medial midbrain, substantia nigra, red nucleus, oculomotor and trochlear nuclei, oculomotor nerve, reticular formation, and posterior internal capsule. Thalamoperforator compromise can cause somatesthetic disturbances, weakness, memory deficits, autonomic imbalance, diplopia, alterations of consciousness, and endocrine disturbances. The peduncular and thalamogeniculate perforators arise from the P2 segments and also ascend superiorly. The long and short circumflex perforators originate from the P1 and P2 segments but they course parallel and medial to the PCA rather than ascending. The medial posterior choroidal artery originates from the P1 segment, and its course resembles that of the circumflex arteries, traveling through crural, ambient, and quadrigeminal cisterns, coursing over the superior colliculus and pineal gland to reach the choroid plexus in the roof of the third ventricle and the floor of the lateral ventricles. In contrast to the posterior thalamoperforators, the anterior thalamoperforators arise from the PCoA along its superior and lateral surfaces. These “premamillary” arteries course superiorly to the floor of the third ventricle in front of the mamillary bodies and supply the posterior hypothalamus, anterior thalamus, posterior limb of internal capsule, and subthalamus. The PCoA itself can have normal anatomy and complete the posterior half of the circle of Willis. The PCoA has a reciprocal relationship with the P1 segment; diminutive or absent PCoAs are associated with large P1 segments, whereas fetal PCoAs are associated with hypoplastic P1 segments (Fig. 19.2). Fig. 19.1 Microsurgical anatomy of the basilar artery (BA) apex and posterior cerebral artery (PCA). Inferior (A) and lateral (B) views of the basilar quadrifurcation and the PCA segments: P1, precommunicating segment; P2, post-communicating segment (P2A, crural segment, and P2P, ambient segment); P3, quadrigeminal segment; and P4, calcarine segment. The superior cerebellar artery (SCA) also divides into four segments: s1, anterior pontomesencephalic segment; s2, lateral pontomesencephalic segment; s3, cerebellomesencephalic segment; and s4, cortical segment. AICA, anterior inferior cerebellar artery; AmbC, ambient cistern; CruC, crural cistern; ICA, internal carotid artery; MCA, middle cerebral artery; PCoA, posterior communicating artery; PICA, posterior inferior cerebellar artery; QuadC, quadrigeminal cistern; VA, vertebral artery. Fig. 19.2 (A) Classic anatomy with large, symmetrical PCoAs completing the posterior circle of Willis. The PCoA has a reciprocal relationship with the P1 segment: (B) a fetal PCA is associated with a hypoplastic P1 segment, and (C) a diminutive or hypoplastic PCoA is associated with a large P1 segment. (D) Posterior thalamoperforators arise from the P1 segments, with the majority arising from the middle third as individual branches or branching trunks that can be bilateral and symmetric, (E) bilateral and asymmetric, or (F) unilateral with bilateral territory (artery of Percheron). Pit, pituitary. Basilar bifurcation aneurysms are approached from the right side to avoid the dominant hemisphere, unless the aneurysm anatomy favors a left-sided approach, left oculomotor palsy already exists, right hemiparesis already exists, or additional left-sided aneurysms will also be clipped. The orbitozygomatic approach is used routinely, and microdissection begins with a wide splitting of the sylvian fissure to expose the middle cerebral artery (MCA) bifurcation and the M1 segment. The lamina terminalis is fenestrated early to relax the brain. The view of the basilar apex from a transsylvian perspective is blocked by the optic nerve and the supraclinoid internal carotid artery (ICA). Three anatomic triangles provide a view through or around these obstacles: the optic-carotid triangle, the carotid-oculomotor triangle, and the supracarotid triangle (Fig. 19.3). The optic-carotid triangle is bordered medially by the optic nerve, laterally by the supraclinoid ICA, and posteriorly by the A1 segment. It provides a medial-superior view of the pituitary stalk, mamillary bodies, interpeduncular fossa, and high-riding aneurysms. This triangle is small and confining, with limited maneuverability. The supracarotid triangle lies above the ICA bifurcation, bordered by the A1 segment medially, the M1 segment laterally, and the basomedial frontal lobe superiorly. This triangle provides a view of the basilar apex when the supraclinoid ICA is short, atherosclerotic, or difficult to retract medially, but it is obstructed by passing perforators from the ICA bifurcation that supply the anterior perforated substance, caudate nucleus, putamen, globus pallidus, superior half of the internal capsule, and anterior thalamus. The carotid-oculomotor triangle is bordered by the ICA medially, the oculomotor nerve laterally, and the uncus posteriorly. It is the largest of the three triangles and can be widened by retracting the ICA medially, working lateral to the oculomotor nerve, and mobilizing the temporal lobe posteriorly. This triangle provides the best panorama of the basilar apex. Fig. 19.3 Anatomic triangles providing access to the basilar bifurcation: 1, optic-carotid triangle; 2, carotid-oculomotor triangle; and 3, supracarotid triangle. The carotid-oculomotor triangle is the one used most commonly for basilar bifurcation aneurysms. Tent., tentorium. The anterior temporal lobe covers the operative corridor through the carotid-oculomotor triangle, and 2 to 3 cm of the temporal lobe retraction posteriorly uncovers it. The temporal lobe retracts naturally in a posterolateral direction if it is detached on all sides (Fig. 19.4). The superior temporal lobe is separated from the frontal lobe by splitting the sylvian fissure (Fig. 19.4, step 1); the inferior temporal lobe is freed from the middle fossa floor by cutting the arachnoid adhesions, dividing any bridging veins, and cauterizing the arachnoid granulations (Fig. 19.4, step 2); and the anterior temporal lobe is untethered by sacrificing the vein to the sphenoparietal sinus (Fig. 19.4, step 3). Systematic release of these attachments allows the temporal lobe to retract posterolaterally (Fig. 19.4, step 4). The inferior edge of the craniotomy will not impede lateral retraction if it has been properly drilled down to the middle fossa floor. The remaining attachments along the medial temporal lobe are released by dissecting the anterior choroidal artery (AChA) along its cisternal segment (Fig. 19.4, step 5). After originating from the ICA, the AChA courses along the medial uncus, follows the optic tract to the lateral aspect of the cerebral peduncle, and enters the crural cistern. AChA dissection, therefore, opens the tentorial incisura and crural cistern, identifies the P2 segment, liberates the oculomotor nerve, exposes the cerebral peduncle, and, in the process, releases the medial temporal lobe. A temporal lobe retractor positioned with its tip on the uncus and its blade against the lateral temporal lobe widens the carotid-oculomotor triangle posteriorly and opens a pretemporal corridor through the middle cranial fossa. Fig. 19.4 Superficial dissection strategy for basilar bifurcation aneurysms. Detaching and mobilizing the anterior temporal lobe opens the operative corridor through the carotid-oculomotor triangle. (A) Splitting the sylvian fissure separates the frontal and temporal lobes (step 1); cutting arachnoid adhesions and granulations along the middle fossa floor frees the inferior temporal lobe (step 2); and dividing the temporopolar vein untethers the anterior temporal lobe (step 3). (B) The temporal lobe now mobilizes posterolaterally, opening the pretemporal corridor (step 4). Dissection along the cisternal segment of the anterior choroidal artery (AChA) releases the medial temporal lobe (step 5). Dissection along the anterior temporal artery (ATA) allows more posterior mobilization of the temporal lobe (step 6). Fig. 19.5 Deep dissection strategy for basilar bifurcation aneurysms. Step 1, identifying the PCoA as it originates from the ICA; steps 2 and 3, following the PCoA to the P1–P2 junction; step 4, dissecting the P2 segment laterally over the oculomotor nerve to the tentorial edge; step 5, dissecting the inferior surface of the P1 segment medially through Liliequist’s membrane; step 6, identifying the SCA; and step 7, securing proximal control on the basilar trunk. An, aneurysm. Temporal lobe retraction can avulse the anterior temporal artery (ATA) from the M1 segment. Dissection along the ATA relieves retraction tension on this artery and allows more posterior mobilization of the temporal lobe (Fig. 19.4, step 6). Occasionally it may require sacrifice. The ATA supplies a silent territory in the nondominant hemisphere and can be occluded without sequelae, whereas an avulsed ATA can injure the M1 segment and cause ischemic complications. Dissection down to the carotid-oculomotor triangle begins by identifying the PCoA as it originates from the ICA (Fig. 19.5, step 1) and following it to the P1–P2 junction (Fig. 19.5, step 2 and 3). The P2 segment is dissected laterally over the oculomotor nerve out to the tentorial edge to open the posterior portion of the triangle (Fig. 19.5, step 4). When anatomic landmarks are buried in clot from the aneurysm rupture, the same steps are taken and the clot is carefully evacuated to excavate these guiding arteries. After one has identified the posterolateral borders of the carotid-oculomotor triangle, the P1 segment is followed proximally to Liliequist’s membrane, and the interpeduncular cistern is opened (Fig. 19.5, step 5). This dense arachnoid sheet extends from the dorsum sellae to the mamillary bodies and between the oculomotor nerves, separating chiasmatic and interpeduncular cisterns. Liliequist’s membrane is opened with an incision behind the posterior clinoid process and medial to the oculomotor nerve, extending posteriorly to the undersurface of the P1 segment, where it pierces the membrane. This incision avoids anteriorly projecting aneurysms that might be encountered with a more medial incision. It also leads to the undersurface of the P1 segment, which is the safe surface to follow medially to the SCA and the basilar trunk because it avoids the thalamoperforators and the aneurysm neck on the upper surface. The SCA lies below the oculomotor nerve and is crossed carefully with the incision in Liliequist’s membrane (Fig. 19.5, step 6). A short segment of the basilar trunk is secured for proximal control (Fig. 19.5, step 7). Numerous perforators originate from the distal basilar artery, but proximal dissection leads to a perforator-free zone below the SCA origins. Subarachnoid hemorrhage (SAH) may bury arteries in clot, and the arteries are excavated along their course proximal to the aneurysm to avoid the dome. Anteriorly projecting aneurysms may require a deeply descending route to the proximal basilar artery to avoid the dome. Proximal control enables dissection to return to the P1 segment, and its superior surface is traced proximally to the aneurysm neck (Fig. 19.6, step 8). The PCoA and its anterior thalamoperforators sweep superiorly to open the medial corridor. A small PCoA that tethers the P1 segment or compromises the view can be cauterized and divided if its anterior thalamoperforators can be preserved; the PCoA cannot be sacrificed with fetal anatomy or when permanent clipping is expected to compromise antegrade flow in the P1 segment. When the aneurysm neck is found, dissection shifts across the basilar apex to the contralateral SCA and PCA (Fig. 19.6, steps 9 and 10), saving perforator dissection for last. The contralateral SCA lies in the line of sight through the carotid-oculomotor triangle, and the contralateral oculomotor nerve is an orienting landmark that differentiates the SCA (below the nerve) from the PCA (above the nerve). Identification of the five major arteries (the basilar trunk, the bilateral PCAs, and the bilateral SCAs) gives the neurosurgeon vascular control, anatomic orientation, and clearance to dissect the aneurysm neck and thalamoperforators. The pathway across the anterior neck is easily visualized in the operative corridor (Fig. 19.6, step 11). The pathway across the posterior neck is not easily visualized and is saved for the final step, often with temporary clipping and mobilization of the aneurysm (Fig. 19.6, step 12). Fig. 19.6 Deep dissection strategy for basilar bifurcation aneurysms. Step 8, dissecting the ipsilateral P1 segment along its superior surface proximally to the aneurysm neck; step 9, shifting across the basilar apex to identify the contralateral SCA; step 10, identifying the contralateral PCA and distal aneurysm neck; step 11, clearing a pathway across the aneurysm neck for the anterior clip blade; step 12, dissecting perforators across the posterior aneurysm neck for the posterior clip blade. Visualization of perforators in the blind spot (X) often requires temporary clipping and aneurysm mobilization. PCP, posterior clinoid process. The contralateral P1 segment, the distal aneurysm neck, and the deep thalamoperforators are difficult to dissect because space in the interpeduncular cistern and exposure at the depth of the surgical corridor are so limited. Aneurysm projection conceals this anatomy further, depending on projection superiorly, anteriorly, or posteriorly. Superior dome projection is most common, and these aneurysms hide deep perforators behind the distal neck (Fig. 19.7A). The contralateral PCA and SCA are usually seen well with an anterior view facing the quadrifurcation. Anterior dome projection is least common, and these aneurysms hide the contralateral PCA and SCA (Fig. 19.7B). The neck is usually seen by working above and behind the aneurysm from the ipsi- to contralateral PCA. The thalamoperforators course away from the dome and are easier to dissect than with other basilar bifurcation aneurysms. Posterior dome projection hides the thalamoperforators, displacing them posteriorly and plastering them to the back wall, making this aneurysm the most difficult (Fig. 19.7C). The quadrifurcation is in full view, but the neck lies behind it and out of view. Many aneurysms have a dome projection that is mixed, like the large aneurysm that points superiorly but also has a posterior “rump” that can displace thalamoperforators. Final dissection hones in on the blind spots unique to each aneurysm.
 Microsurgical Anatomy
Microsurgical Anatomy
 Aneurysm Dissection Strategy
Aneurysm Dissection Strategy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree