Cohort 1
Cohort 2
Hypoperfusion
Cerebral Infarction
Population
Aneurysmal SAH
Aneurysmal SAH
Inclusion criteria
15O-PET performed within 1 day of angiogram
Both postprocedure and predischarge CT (at least 7 days after SAH) performed
Assessment of angiography
Quantitative measurement of proximal stenosis, qualitative assessment of distal vessels
Qualitative assessment by neuroradiologist (retrospective)
Vasospasm defined as
Stenosis ≥50 % or moderate-severe distal narrowing
Moderate-severe in proximal and/or distal vessels
Radiographic measurement
Cerebral blood flow measured in 28 brain regions by PET
Cerebral infarct seen on final CT after exclusion of early/postprocedural infarcts
Hypoperfusion: CBF < 25 ml/100 g/min
Number of patients
25
134
Poor Grade (WFNS IV-V)
10 (40 %)
42 (31 %)
Modified Fisher Grade 3-4
23 (92 %)
119 (89 %)
Clip/Coil
15/10
67/67
Angiographic vasospasm in
14 (56 %)
54 (34 %)
Death or discharge to LTC
3 (12 %)
18 (13 %)
Results
Relationship of Vasospasm and CBF
PET was performed a median of 6 h from time of angiography in this cohort of 25 patients. Fourteen patients had moderate-severe CVS in at least one vascular territory. Although 12 of the patients had symptomatic DCI at some point in their course, only 3 patients were being actively treated with hypertensive therapy at time of PET. Excluding these patients, mean arterial blood pressure was not different between those with and those without CVS. Twenty-four percent of all brain regions were affected by significant (proximal and/or distal) CVS, involving 34 vascular territories across the 14 affected patients. Global mean CBF tended to be lower in patients with CVS (38.4 ± 9.7 vs. 44.3 ± 14.5 ml/100 g/min in those without CVS, p = 0.07). Regional CBF was also lower (mean 38.6 ± 11.7) in regions affected by CVS compared with 48.7 ± 16.5 ml/100 g/min in brain regions without CVS (p < 0.001). A graded reduction in CBF was seen between mild, moderate, and severe degrees of CVS (Fig. 1). This reduction in perfusion was present whether there was only proximal, only distal, or both proximal and distal CVS present.
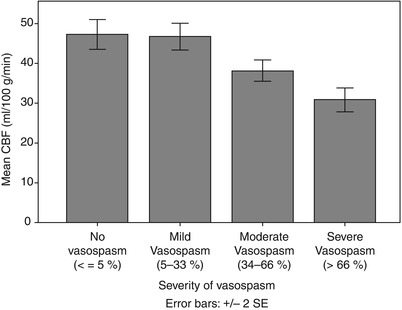

Fig. 1
Mean regional CBF in territories with varying severities of cerebral vasospasm (excluding three patients on vasopressors)
More relevant to the question at hand, however, regional hypoperfusion (rCBF < 25 ml/100 g/min) was not confined or even preferentially located within territories affected by CVS. We found regions with low CBF in 10 of 25 patients, including 7 of 14 patients with CVS but also 3 of 11 patients without significant CVS. Nine of these ten patients with hypoperfusion had neurological symptoms during their course, but none were on vasopressors at the time of PET. Interestingly, two of the three patients with hypoperfusion but no CVS still had neurological deficits. A total of 46 brain regions exhibited hypoperfusion (CBF < 25 ml/100 g/min) across ten patients. Regional hypoperfusion did not occur more frequently in territories with significant CVS (15 of 157 affected regions, 10 %) vs. 20 of 217 (9 %) of unaffected regions, even when restricting analysis to only patients with CVS (i.e., comparing territories with and without vasospasm in the same group of patients). Therefore, the majority of regions with hypoperfusion were found outside territories with CVS. In fact, 54 % of hypoperfused regions were located in vascular territories with no CVS anywhere in the ipsilateral carotid (or, if appropriate, vertebrobasilar) circulation. When the 11 patients without CVS were also included, a total of 31 of the 46 (67 %) hypoperfused regions were not in the territory of any affected vessels (Fig. 2). This relationship persisted even after excluding the three patients on vasopressors, in whom treatment could have masked an association between vasospasm and hypoperfusion.
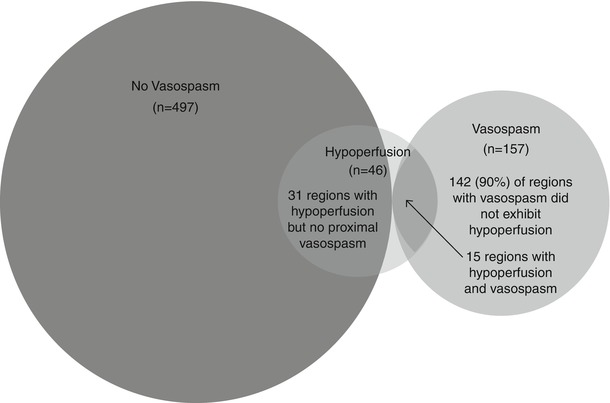

Fig. 2
Venn diagram of brain regions within territories affected and unaffected by cerebral vasospasm, overlapping with regions exhibiting hypoperfusion (CBF < 25 ml/100 g/min)
Relationship of Vasospasm to Delayed Cerebral Infarction
Delayed cerebral infarcts were seen in 20 of the 134 patients (15 %) after exclusion of early/procedural infarcts (which accounted for an equivalent proportion of the total infarcts); moderate-severe angiographic CVS was present in 34 % of this cohort. The rate of delayed infarction was higher in those with CVS compared with those without CVS (31 % vs. 4 %, p < 0.001), although in one of these patients with CVS and infarction, the infarct actually occurred in a vascular territory without CVS. Fifteen of the 20 patients with delayed infarcts had infarcts in territories with CVS, 4 had infarcts unrelated to CVS, and 1 patient had both infarcts related and unrelated to vasospasm. There were no patient characteristics that differentiated these groups, although the number of patients in each group was admittedly low. There were a total of 29 delayed cerebral infarcts, of which 21 were associated with corresponding angiographic CVS, whereas 8 (28 %) occurred in the absence of any proximal CVS. Half of the vasospasm-related infarcts were in the ACA territory whereas no infarcts unrelated to CVS were in the ACA region. Rather, half the vasospasm-unrelated infarcts occurred in watershed territories, compared with 10 % of infarcts seen with CVS (p = 0.03). There was no difference in volume of these two infarct types and they were evenly divided between cortical and subcortical regions.
Discussion
Vasospasm and Hypoperfusion
We demonstrated that CVS is associated with lower global CBF in affected patients as well as lower regional CBF within affected territories. Still, this association does not confirm a causal or pathophysiologic link between radiographic narrowing and hypoperfusion. It may be that other processes coexist in those with CVS, and it may be these that are primarily responsible or contribute concurrently to reductions in CBF. For example, those at high risk for angiographic CVS are also more likely to develop microcirculatory dysfunction, and the latter could also contribute to tissue hypoperfusion. Our main finding that cerebral hypoperfusion occurs as commonly in the absence of proximal CVS as in its presence further questions the direct relationship between CVS and CBF. Even for patients who had some territories with CVS, hypoperfusion was equally likely to be found in brain regions located outside territories with angiographic abnormality. A similar discordance was found in a study that compared transcranial Doppler (TCD)-defined CVS with PET measurements of hemispheric CBF [10]. Such findings suggest that hypoperfusion (and therefore likely ischemia/DCI) are mediated in significant part by processes other than angiographic CVS alone. These may include distal microcirculatory abnormalities that reduce tissue perfusion, such as impaired vasodilation, microvascular thrombosis, capillary endothelial dysfunction, and cortical spreading depolarizations [13].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





