Fig. 17.1
Histological and behavioral evaluation after anti-interleukin (IL)-6 receptor (MR16-1) treatment. Luxol fast blue (LFB) stained axial sections at the injury epicenter (42 days post-injury) showed remarkable reduction in the area of demyelination in the MR16-1-treated group (a, b), greater abundance of growth-associated protein (c–f), and neurofilament heavy 200 kDa-positive nerve fibers (g–j) compared with rat IgG control group. (k) Basso Mouse Scale (BMS) score showed significant hind limb motor function improvement in the MR16-1-treated group. Scale bar (a, b) 200 μm, (c, d, g, h) 500 μm, (e, f, i, j) 50 μm. (c–f) GAP-43 conjugated to Alexa Fluor 568 (red); (g–j) NF-H conjugated to Alexa Fluor 568 (red) (Reprinted, in part, with permission, from [21])
The contusive SCI resulted in immediate complete paralysis. At 7 days post-injury, the MR16-1-treated group showed a significant improvement in Basso Mouse Scale (BMS) locomotor score; this difference continued up to 6 weeks post-injury (Fig. 17.1k). At that point, the BMS score of the MR16-1-treated group was 5.0 ± 0.3, whereas that of the control group was 2.8 ± 0.3 points.
17.1.2 MR16-1 Treatment Reduced Th1 Cytokine and Increased Th2 Cytokine Levels at Injury Site After SCI
Using immunoblot analysis (Fig. 17.2), we evidenced that IFN-γ levels (28 kDa band) (Fig. 17.2a) and TNF-α levels (19 kDa band) were markedly reduced in the MR16-1-treated group from 1 to 14 days after injury (Fig. 17.2b), with increased protein levels of IL-4 (18 kDa band) reaching a peak at 7 days post-injury (Fig. 17.2c) as well as IL-13 levels (13 kDa band) (Fig. 17.2d).
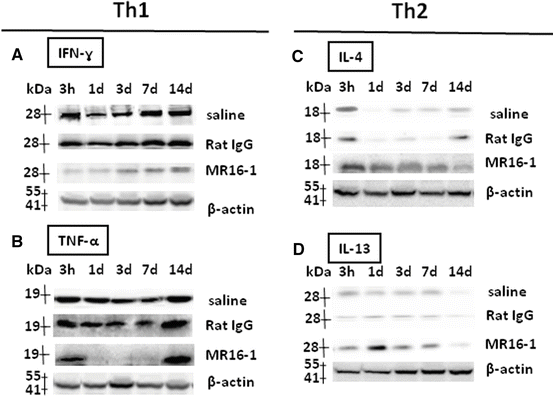

Fig. 17.2
Immunoblot analysis of T helper (Th)1 and Th 2 cytokines after MR16-1 treatment. Compared with control groups, MR16-1-treated group samples showed decreased levels of interferon (IFN)-γ (a) and tumor necrosis factor (TNF)-α (b), with increased expression of interleukin (IL)-4 (c) and IL-13 (d). Band intensity normalized to that of β-actin (Reprinted, in part, with permission, from [21])
17.1.3 MR16-1 Treatment Promoted M1 Macrophages Decrease and M2 Macrophages Increase at Lesion Site After SCI
Rat IgG control group showed larger lesion sites with cells extending from the epicenter (in posttraumatic hematoma) in contrast to MR16-1-treated group (Fig. 17.3a–d). Greater numbers of iNOS-positive cells at 3 days post-injury were found in the rat IgG control group (Fig. 17.3e–h). By contrast, arginase-1-positive cells at 3 days post-injury populated the lesion site in the MR16-1-treated group (Fig. 17.3k, l) in higher numbers than in the control group (Fig. 17.3i, j). The patterns of immunopositivity for iNOS and arginase matched the distribution of CD11b-positive cells (activated macrophages).
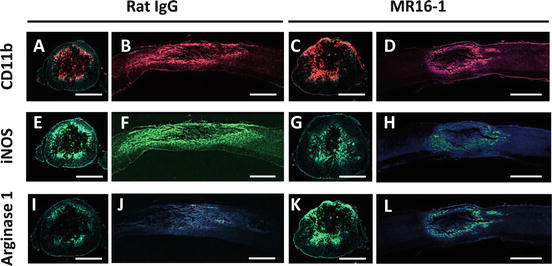

Fig. 17.3
Distribution of classically activated (M1) and alternatively activated (M2) phenotype macrophages in the injured spinal cord at 3 days after MR16-1 treatment. MR16-1-treated group samples showed smaller injury sites with centralized presence of CD11b-positive cells (a–d). Inducible nitric oxide synthase (iNOS)-positive cells were predominant and distributed over the same CD11b-positive area in the rat IgG control group, (e–h), whereas MR16-1-treated group showed larger numbers of arginase-1-positive cells, localized more specifically to the injury site (i–l). (a–d) CD11b conjugated to Alexa Fluor 568 (red); (e–h) iNOS conjugated to Alexa Fluor 488 (green); (i–l) arginase-1 conjugated to Alexa Fluor 488 (green); (a–l) DAPI used for nuclear counterstaining (blue). Scale bar: (a, c, e, g, i, k) 200 μm, (b, d, f, h, j, l) 500 μm (Reprinted, with permission, from [21])
At the lesion epicenter, the MR16-1-treated group showed decreased numbers of iNOS- or CD16/32-expressing macrophages with increased populations of arginase-1- or CD206-expressing macrophages, up to 14 days post-injury.
17.1.4 MR16-1 Treatment Inhibited Interferon (IFN)-γ Production by Neutrophils and Increased Interleukin (IL)-4 Expression in Microglia and Macrophages
Flow cytometry analysis showed significantly lower numbers of IFN-γ-positive neutrophils infiltrating the injured SC from 1 to 7 days after injury in the MR16-1-treated group (Fig. 17.4a, b).
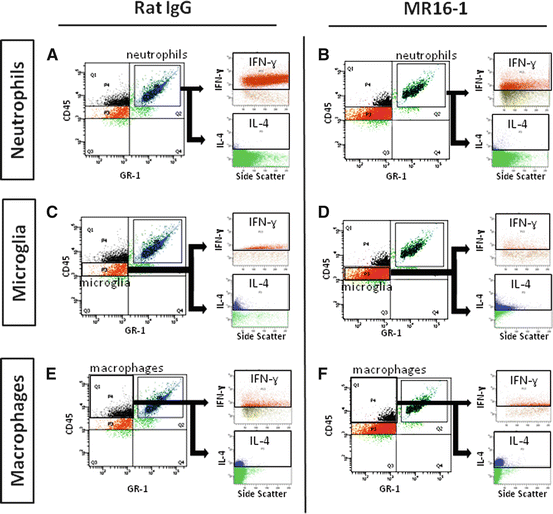

Fig. 17.4
Semiquantitative flow cytometry analysis of interferon (IFN)-γ and interleukin (IL)-4 levels in neutrophils, microglia, and macrophages after MR16-1 treatment. Flow cytometry plots at 1 day post-injury showed reduction of neutrophils positive for intracellular IFN-γ in MR16-1-treated group (a, b). At 3 days post-injury, MR16-1-treated group samples had larger numbers of IL-4-positive microglia (c, d) and reduced total number of macrophages with increased IL-4 expression and decreased IFN-γ expression (e, f) (Reprinted, in part, with permission, from [21])
Significantly larger numbers of IL-4-positive microglia were present in the MR16-1-treated group at 3 days after injury (Fig. 17.4c, d). The number of macrophages infiltrating the SC after injury was reduced in the MR16-1-treated group compared with the rat IgG control group (Fig. 17.4e, f); such macrophage population showed increased intracellular IL-4 levels at days 1 and 3 after injury, with decreased intracellular IFN-γ levels at 3 days after injury.
17.1.5 MR16-1 Treatment Changed the Predominant Phenotype of Macrophages and Their Abilities After SCI
Flow cytometry analysis showed that MR16-1 treatment was associated with a marked shift from an iNOS-positive to arginase-1-positive macrophage population, from 1 to 7 days post injury, and from CD16/32-positive to CD206-positive macrophage population, from 3 up to 14 days after injury (Fig. 17.5a, b), matching the results of immunostaining.
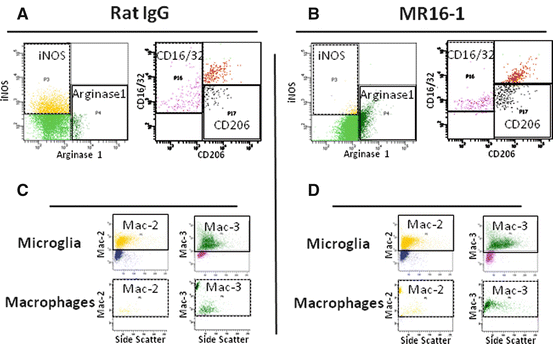

Fig. 17.5
Semiquantitative flow cytometry analysis of phagocytic and digestive activities of alternatively activated macrophages after MR16-1 treatment. (a, b) Three days post-injury: MR16-1-treated group showed lower numbers of iNOS-positive and CD16/32-positive macrophages with an increased arginase-1-positive and CD206-positive macrophage population. (c, d) Microglia of both groups showed no difference in Mac-2 and Mac-3 expression; however, the number of Mac-2- and Mac-3-positive cells within the population of arginase-1-positive macrophages was significantly larger in the MR16-1-treated group than in the rat IgG control group (Reprinted, in part, with permission, from [21])
Arginase-1-positive macrophages of the MR16-1-treated group had enhanced positivity for Mac-2 and Mac-3 compared with the rat IgG control group, and these differences were significant from 1 up to 7 days post-injury (Fig. 17.5c, d).
17.2 Discussion
IL-6 is a multifunction cytokine crucial for T-/B-cell differentiation and proliferation, immunoglobulin secretion, acute phase protein production, and macrophage/monocyte function [22]. In the pathophysiology of SCI, IL-6 is considered to be a pro-inflammatory cytokine that triggers secondary injury [10]. Once IL-6 is released, it binds to the membrane-bound IL-6R in an IL-6/IL-6R complex that associates with gp130 to exert a signal into cells [13]. MR16-1 is a rat anti-mouse IL-6R antibody that competitively inhibits the binding of IL-6 to IL-6R dose dependently, has a half-life of about 3 days in mice, and exhibits anti-inflammatory properties in rheumatoid arthritis [23] and SCI [13, 14]. In the present study of SCI, the MR16-1-treated group had smaller injury sites with less connective tissue formation secondary to the blockade of reactive astrogliosis reported previously [14]. Increased myelin sparring and an enhanced SC repair process, as shown by an increased prevalence of NF-H-positive and GAP-43-positive fibers at 42 days post-injury, were probably the consequence of increased neuronal survival in response to a diminished inflammatory cascade [24]. In addition, MR16-1 treatment improved locomotor BMS score from 7 days after SCI compared with the control groups, suggesting anatomical improvement, as reported by previous researchers [14, 15, 24]. We also found that MR16-1 treatment reduced the levels of the Th1 cytokines IFN-γ and TNF-α, with a parallel increase in levels of the Th2 cytokines IL-4 and IL-13 at the site of the spinal lesion in the acute phase after SCI. Hence, we hypothesized that a temporal blockade of IL-6 signaling by MR16-1 treatment changed the profile of cytokines present in the injured SC into an alternative macrophage-activating environment. In agreement with previous research, significant increases in IL-4 and IL-13 levels [7, 9, 25] and simultaneous reduction in IFN-γ and TNF-α levels would promote the formation of alternatively activated macrophages and inhibit that of the classically activated macrophages [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








