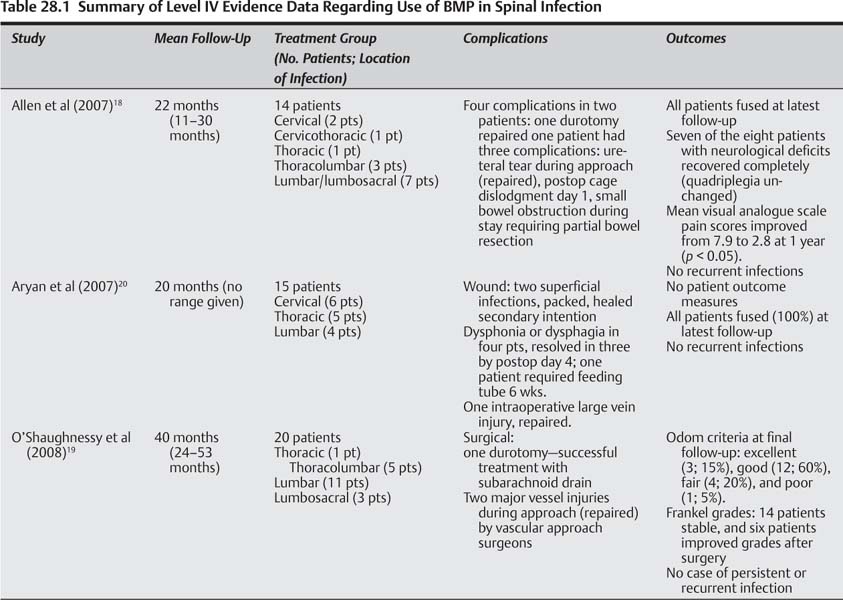
28 The incidence of pyogenic spinal infection is increasing,1–6 occurring in as many as 1/100,000 individuals annually.7,8 The vertebrae are the most common sites for hematogenously acquired osteomyelitis.1–9 Vertebral osteomyelitis may account for up to 7% of all bony infections.1–9 Several factors underlie increasing rates of spinal infection, including an increasing elderly population, higher prevalence of patients with chronic immunosuppressive diseases, and an increasing number of spinal procedures performed in North America each year10–12 with rates of instrumented lumbar fusion increasing 20-fold in some regions in the past several years.10–12 Though magnetic resonance imaging (MRI) may facilitate earlier diagnosis,13–15 vertebral osteomyelitis commonly presents in delayed fashion, predominantly with pain. Systemic symptoms and signs of illness may be present, such as fever, chills, night sweats, and elevated laboratory markers of infection [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR)]. Spinal infection is most commonly due to Staphylococcus aureus, accounting for 40 to 80% of all spinal infections.8,16 Less common causes include gram-positive organisms such as S. epidermidis and Streptococcus.8,16,17 Untreated or medically unresponsive pyogenic vertebral infection often becomes progressive, leading to instability, neurological deterioration, intractable pain, deformity, and/or death.1,2 Stable patients without progressive neurological deficit or instability can be treated by identification of the causative organism (s) followed by appropriate antibiotic therapy, often supplemented by a spinal orthosis. However, up to 50% of patients fail nonoperative measures1–5 and require surgical debridement and spinal stabilization. In general, surgical indications include real or impending spinal instability, spinal deformity, need to establish a diagnosis/negative percutaneous biopsy, progressive pain, neurological deficit, sepsis refractory to broad-spectrum antibiotics, or those who have failed appropriate organism-directed antibiotic therapy. Historically, attempts to clear spinal infections, both non-surgically and surgically, were fraught with relatively poor outcomes, and relatively high rates of perioperative morbidity, complications, infection recurrence, and mortality rates. Recent advances in medical care, antibiotics, anesthesia, and surgical techniques, including advanced spinal instrumentation, have increased success rates of infection clearance and improved outcomes.1–6,18–20 Successful surgical strategies for pyogenic spinal infection include the use of posterior segmental (titanium) pedicle screws and anterior titanium cages, with autograft iliac crest and/or allograft bone (structural or nonstructural), often at the time of primary debridement of the active infection.1–5,17,21–25 Though titanium instrumentation in the infected spine has shown increased rates of clinical and radiographic success, obtaining fusion can be difficult in patients with more extensive disease and comorbidities. In these cases, in particular, graft options remain limited.1–5,16–21 In addition to autograft17,25–27 and allograft,21,28 vascularized interposition grafts (e.g., pedicled rib flaps, free flaps)19 are another graft type that may be used, with relatively good reported success rates. Though iliac crest bone graft (ICBG) may be ideal, prior harvest, need to fix to the pelvis, and limited graft quality, all limit the potential quantities and utility of this type of graft, especially in severe, multilevel cases. In addition, complications still exist with these graft options, including pseudarthroses,7,16,22,29 donor site morbidity,16 recurrence or persistence of spinal infection,24 and death.1–5,22,24,29,30 This has prompted some to explore the role of a recombinant biologic in their surgical treatment regimen in anticipation of forming a solid fusion with fewer graft-related morbidities. Bone morphogenetic proteins (BMPs) are part of the transforming growth factor-β (TGF-β) superfamily of growth factors, which regulate multiple cellular functions, including inflammation, cell growth, differentiation, migration, and apoptosis.31 Members of the TGF-β superfamily are known to modulate immune responses to multiple types of infections by controlling inflammation and repair after injury.32 The osteoinductive capabilities of BMPs have been studied extensively since their identification by Marshall Urist in 1965.33 Recombinant human BMP-2 (rhBMP-2) has been available for general use since July 2002, and is currently U.S. Food and Drug Administration (FDA) approved for: anterior lumbar interbody fusion (ALIF), placed in a specific titanium cage, from L2–S1 in skeletally mature patients with degenerative disk disease (DDD); certain oral maxillofacial and dental regenerative uses (sinus augmentations, localized alveolar ridge augmentations for defects associated with extraction sockets), and acute open tibial shaft fractures stabilized with an intramedullary nail (must be applied within 14 days of the fracture). While deemed safe and effective by the FDA for the aforementioned indications, in studies ranging from case series to higher level I and II studies, rhBMP-2 has been shown to be an effective osteoinductive agent for a multitude of non-FDA-approved (“off-label”) applications. For example, excellent fusion rates and good clinical results have been reported when using rhBMP-2 in single-level instrumented lumbar fusions and in posterior lumbar fusions in patients over 60 years of age.6,34–36 Though this rather seamless transition of rhBMP-2 use in non-FDA approved applications for spinal fusions has occurred, it is not without complications, such as the dose-related swelling issues with use in the cervical spine. Therefore, use of rhBMP-2 in human spinal infection should not be considered a forgone conclusion. High-level studies supporting the osteoinductive abilities of rhBMP-2 bring with them important observations regarding infection during the use of rhBMP-2. In ALIF with tapered cages or allograft for degenerative disk conditions, rhBMP-2 use resulted in fusion rates superior to iliac crest autograft (e.g., allograft/rhBMP-2 99% versus allograft/ICBG 76%).6,37 In open tibia fractures, rhBMP-2 use led to faster fracture union and wound healing, fewer implant failures, fewer subsequent procedures, and, importantly, fewer infections than controls.38 However, the association of rhBMP-2 use in open tibia fractures with decreased infection rates does not necessarily imply that rhBMP-2 is osteoinductive during active acute or chronic infection in humans, or that it decreases local spread of infection.18,39,40 Use of rhBMP-2 in active infections is not FDA approved. In fact, the package insert (as required by the FDA) lists active infection as a contraindication to rhBMP-2 use.41 This may be related to the lack of studies on the use of rhBMP-2/sponge carrier in infection, and the concern that infection could be enhanced. Interestingly, the same package insert lists infection as a “potential adverse event” of rhBMP-2. Based on the combined INFUSE plus LT-CAGE device data, as listed within the adverse events table of the package insert, 19 patients (rhBMP-2 group) had infections versus only nine in the control group from postoperative day 1 to 4 weeks; at 4 to < 9 weeks eight in the rhBMP-2 group versus four in the control group had infections. Taken together, a lower infection rate was seen when rhBMP-2 was used in open tibia fractures, whereas a higher infection rate (not statistically significant) was found in ALIFs, particularly within the first 8 weeks following surgery. One way to help determine the utility of rhBMP-2 use in human infection, albeit indirect, is to analyze the infection rates in humans following rhBMP-2 use, to see if they are lower than expected. In multicenter, prospective randomized studies on the use of rhBMP-2 for maxillary sinus floor augmentation, the authors found significantly less edema, less pain, and an almost 45% lower infection rate with rhBMP-2 versus autogenous bone graft.42,43 In a 96-patient study of patients reexposed to rhBMP-2 in the lumbar spine, the authors observed no increase in wound infection rates during reexposure.44 During patients’ first exposure to rhBMP-2 there were 90 primary fusions and six revisions with only two wound infections, a lower rate of wound infections than commonly reported for instrumented lumbar spine fusions. However, rhBMP-2 application in posterior cervical spine fusions led to a higher rate of wound infections requiring treatment (6, 14.6%) than the iliac crest autograft group (1, 2.8%), though this did not reach statistical significance (p < 0.113).36 One patient in the ICBG group had a posterior iliac crest wound infection. These results, combined with known anterior cervical complications, suggest that the carrier, concentration of rhBMP-2 used, and in vivo response needs a continued, thorough evaluation before its routine use in these locations. Unfortunately, very few studies (and no randomized trials) have specifically examined the use of BMPs in infection, making the guarded interpretation of evidence presented in this chapter paramount. Several issues must be clarified to determine the utility of BMPs in active (spinal) infection in humans, including (1) What are the indications for BMP-2 use in spinal infection given that its use in the setting of active infection is listed as a relative contraindication by the manufacturer? (2) What is the risk profile associated with the use of BMP-2 in active spinal infection, and has it been found to be different from that previously reported in the literature or by the manufacturer? (3) Is there higher-level scientific evidence for the efficacy of BMP-2 use in the setting of human spinal infection? To address these questions we performed a literature review to determine the best evidence to guide sound clinical decision making on each of the foregoing topics. Search engines included Medline, Embase, and Cochrane Controlled Trials Registry. A search using the term “bone morphogenetic protein” returned 10,742 articles. We then searched by combining “bone morphogenetic protein and infection”—returned 151 articles; “bone morphogenetic protein and spine infection (or vertebral infection)”—returned 20 articles; “bone morphogenetic protein and spine (or vertebral) osteomyelitis”—returned three articles. Initially, the majority of studies were clinical case series or case reports on the use of rhBMP-2 in spine fusions (mainly lumbar, a few anterior or posterior cervical), reporting on outcomes, fusion rates, and associated complications or adverse events (but not infections). Other studies included basic science and mechanistic studies as well as animal studies. There were no level I, II, or III studies that used BMPs in the setting of any infection in humans. There were only three studies, all level IV, that used BMPs (rhBMP-2) in human spinal infection and that were able to provide some meaningful data on the issues discussed in this chapter. A summary of the three studies is provided in Table 28.1. There are no level I, II, or III data. Based on the three level IV studies available, general indications for using rhBMP-2 in active spinal infection were no different than previously reported surgical indications for the treatment of pyogenic vertebral infection. Indications similar to all three studies included persistent back pain/“severe” pain and/or radiculopathy, neurological compromise (including weakness and/or myelopathy), and spinal deformity and/or instability. Subtle differences in indications, mostly a matter of semantics, can be found between the studies. Aryan et al20 included a common finding in vertebral osteomyelitis—MRI positive for “osteomyelitic bone”—in patients with pain, neurological symptoms, and/or spinal deformity as indications. Allen et al18 included severe pain in the presence of epidural or paravertebral abscess, neurological compromise, vertebral destruction, and progressive deformity and/or instability, as well as failure of medical therapies. Similarly, O’Shaughnessy et al19 cited the failure of 4 to 6 weeks of culture-specific antibiotic therapy in all patients, back pain, neurological deficit (Frankel grade), and/or failed prior surgical treatment of osteomyelitis (in 55% of patients) with persistence or recurrence of infection, symptomatic pseudarthrosis, or medically refractory pain with or without spinal deformity. Failed prior surgical treatment for osteomyelitis was more commonly reported as an indication in the study by O’Shaughnessy et al,19 indicating the difficult population being treated. However, it should be noted that symptomatic pseudarthrosis does not necessarily imply that patients had persistent active or chronic infection, but simply a failure to fuse, and may limit the interpretation of some of the data in that study. A comprehensive analysis of each authors’ rationale for rhBMP-2 use in infection provides additional information, and the overlap among the studies helps to guide patient-specific surgical indications. One notable finding was that each article described several patients with either or both severe osteomyelitis and several comorbid conditions. Allen et al18 specifically discussed this difficult patient population in their rationale for using rhBMP-2 and the medically recalcitrant nature of osteomyelitis in their study population, finding that 57% of patients had three or more vertebral bodies involved, and 12 of 14 patients had significant predisposing medical comorbidities. These findings were detailed in a patient-specific fashion within the study tables. All patients in their series underwent circumferential fusion. O’Shaughnessy et al19 also discuss the difficulties treating this population surgically for vertebral osteomyelitis. Eighty percent of their patients required circumferential fusion. Many had failed prior surgical treatment, an important caveat in their study. Importantly, 25% of their patients underwent adjunctive soft tissue coverage procedures at the time of their spinal reconstructions. Flaps in this series included myocutaneous or pedicled omentum. This not only led the authors to suggest that a flap can increase local healing ability by placing vascularized tissue at the site of infection (which may be a result of rhBMP-2 as well), but can help obliterate potential spaces that may retain infection. In addition, the authors suggest that flaps may be a “rich source of mesenchymal stem cells” that may interact with rhBMP-2 to generate bone or fusion. This postulate is theoretical, and the references given in the manuscript deals with an environment completely different than that of an infected, osteomyelitic spine and surrounding soft tissues. In addition, the actions of rhBMP-2 in a soft tissue, or disk-privileged environment, may be different from that in the infected spine. Nonetheless, the theoretical advantages of having a robust source of mesenchymal stem cells, combined with a vascularized flap, may substantially benefit antibiotic delivery and infection clearance. Aside from the foregoing, none of the articles site patient-specific indications for rhBMP-2. Rationales for rhBMP-2 use in spinal osteomyelitis were similar for Allen et al18 and O’Shaughnessy et al.19 Both studies cited animal data on BMP-2 and BMP-7 use in long bone infection, as well as the prospective, randomized, controlled data for rhBMP-2 use in open tibia fractures where patients had a significantly lower rate of infection.38 O’Shaugnessy et al19 discussed the limited availability of autograft in difficult, multilevel cases and in patients with prior iliac crest harvest, whereas Aryan et al20 focused more on instrumenting the infected spine with titanium cages and rhBMP-2 following corpectomy. Allen et al18 discussed using titanium in a single-stage circumferential reconstruction of the infected spine, particularly in difficult-to-treat patients. Though Aryan et al20 discussed their rationale for titanium instrumentation, fusion assessment, patient symptoms, and clearance of infection, the authors did not focus on patient-specific factors in the rationale for rhBMP-2 use. In addition, Aryan et al20 discussed in general terms the safety, costs, and “outcomes” of their patients, but unfortunately no standardized outcome measurement tools were utilized, no pain scores were used, and no marker of neurological integrity or function was utilized (e.g., Frankel grade). Based on the available (level IV) evidence, general indications for using rhBMP-2 in active spinal infection are no different than previously reported surgical indications for the treatment of pyogenic vertebral infection. These indications include persistent or medically refractory back pain/“severe” pain and/or radiculopathy, neurological compromise (including weakness, myelopathy, or paralysis), and spinal deformity and/or instability. Other indications include failure of prior surgical treatment for osteomyelitis, which includes related symptomatic pseudarthrosis and/or persistent or recurrent infection, the need to obtain a biopsy or tissue diagnosis, as well as failure of culture-specific antibiotics and other medical therapies. Pearls • Only three level IV studies have evaluated rhBMP use in humans during active and/or chronic spinal infections. • Three level IV studies suggest that indications for using rhBMP-2 in active human spinal infection are similar to general surgical indications for the treatment of pyogenic spinal infection. • Three level IV studies suggest that preferential consideration should be given to using rhBMP-2 in active human spinal infection in patients that have failed prior surgical treatment for osteomyelitis, have symptomatic pseudarthrosis and/or persistent or recurrent infection, have limited graft options available, have multiple levels involved, or have multiple medical comorbidities.
Bone Morphogenetic Protein in the Setting of Infection: Indications, Risks, and Efficacy
 Bone Morphogenetic Protein in the Setting of Spinal Infection
Bone Morphogenetic Protein in the Setting of Spinal Infection
Indications
Level I, II, III Data
Level IV Data
Summary of data
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree