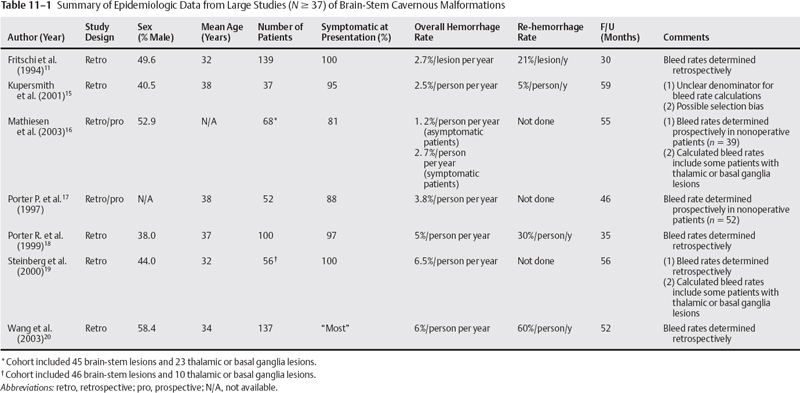
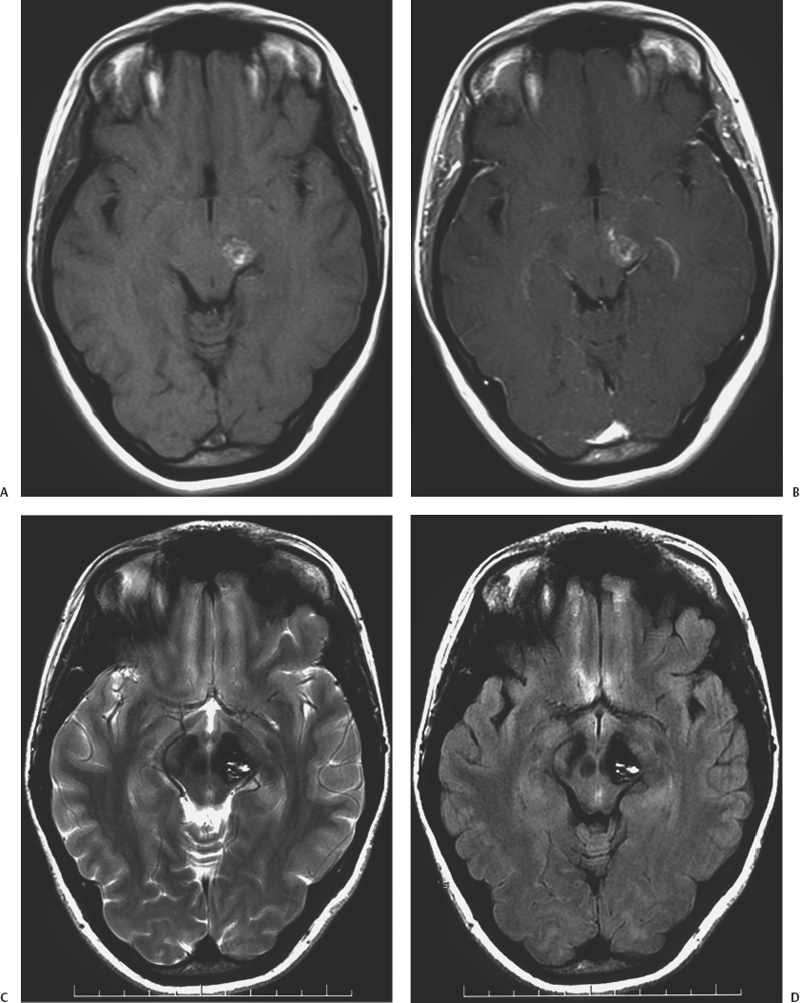
11 Jeffrey D. Klopfenstein, Iman Feiz-Erfan and Robert F. Spetzler Based on autopsy and magnetic resonance imaging (MRI) data,1–7 cavernous malformations (CMs) of the central nervous system (CNS) affect 0.2 to 0.9% of the population. These lesions account for 5 to 16% of CNS vascular malformations.8–10 Of all CNS CMs, 9 to 35% are found in the brain stem accounting for 13% of vascular malformations of the posterior fossa.10–13 Importantly, CMs of the brain stem are more likely than their supratentorial counterparts to become symptomatic and, given their location, to have significant repercussions for affected patients. With recent technological advances (e.g., stereotaxy, neurophysiologic monitoring) and mastery of posterior fossa anatomy, the earlier reluctance to operate on the brain stem has given way to an ever-increasing ability of experienced neurosurgeons to resect brain-stem CMs safely and effectively. However, given the complexity of posterior fossa surgery and its potential sequelae, surgeons must understand the surgical indications for patients with intrinsic brain-stem CMs. This chapter presents the natural history, clinical characteristics, surgical indications, operative techniques, and treatment outcomes for brain-stem CMs as a guide for managing patients with these complex lesions. Over the past decade, several large series focusing on brain-stem CMs have provided important epidemiologic and natural history data (Table 11-1). These series suggest that the age of patients at presentation with symptomatic lesions ranges between 32 and 38 years.11,14–20 Whether one sex is more likely than the other to harbor a brain-stem CMs is unclear. Some studies suggest that the incidence is highest among males,8,18,20,21 whereas others indicate the opposite.13–15,19,22,23 One of the most important issues related to the natural history of brain-stem CMs is their frequency of hemorrhage. The re-hemorrhage rate after an initial hemorrhage is also vital to physicians planning treatment: At presentation, 81 to 100% of patients with brain-stem CMs are symptomatic, most often related to hemorrhage.11,14–20 The important question is whether lesions that have already hemorrhaged at least once have a higher risk of bleeding again compared with those with no history of hemorrhage. Among reported studies, there is little uniformity in terms of how hemorrhage and re-hemorrhage rates have been evaluated. This variability makes it difficult to interpret outcomes. First, some studies are prospective, whereas others are retrospective based on the assumption that the lesions were present since birth. As has been well documented, brain-stem CMs can appear de novo. Therefore, assuming their presence since birth will underestimate the frequency of hemorrhage.24,25 Second, some hemorrhage rates are presented as per person per year, whereas others are reported as per lesion per year. Third, the definition of hemorrhage ranges from a clinical apoplectic event to the definitive presence of acute lesional or extralesional blood on radiographic studies. Finally, almost all large series of brain-stem CMs are from specialized neurosurgical centers; thus, the issue of referral bias arises. Given these limitations, the range of published hemorrhage rates must be considered individually and as a whole to derive a reasonable estimate of hemorrhage and re-hemorrhage rates for brain-stem CMs. Based on published series with more than 20 patients with brain-stem CMs, overall hemorrhage rates have ranged from 2.5 to 7% per patient (or lesion) per year without respect to the number of hemorrhages per patient.11,15–20 (In two studies, hemorrhage rates were based on both brainstem and deep supratentorial lesions ([i.e., thalamus and basal ganglia]; no breakdown was provided although most lesions were in the brain stem).16,19 Mathieson et al. found that patients who presented with symptoms had a hemorrhage rate of 7% per person per year, whereas asymptomatic patients had a rate of 2% per patient per year.16 Porter et al. found that when hemorrhage was defined as an apoplectic clinical event without respect to radiographic findings, the overall hemorrhage rate increased from 3.8 to 10.6% per patient per year.18 Whereas the overall reported rates of hemorrhage are reasonably consistent, the data for re-hemorrhage rates are less consistent. In the large published series, the re-hemorrhage rates for brain-stem CMs with a single previous hemorrhagic episode have ranged from 5.1 to 60% per patient (or lesion) per year.11,15,18,20 Kupersmith et al. reported a re-hemorrhage rate of 5.1%, but the study had major limitations. First, the denominator in the rate calculations was unclear. Second, the series consisted of patients referred to a neuroophthalmology service, suggesting potential bias from a less morbid subgroup compared with other studies.15,26 If this study is excluded from consideration, the published hemorrhage rates vary from 21 to 60% per patient per year. 11,18,20 Despite the limitations, these numbers suggest that lesions that have already hemorrhaged are likely to have an increased risk of subsequent hemorrhage compared with those that have never bled. This likelihood must be considered when selecting patients for surgical management. When the risks of hemorrhage (2.5 to 7%) and re-hemorrhage (21 to 60%) of brain-stem CMs are compared with the hemorrhage (0.25 to 3.1%) and re-hemorrhage (4.5 to 22.9%) rates per patient per year, respectively, for all CNS CMs, brain-stem CMs may be more likely to hemorrhage than their supratentorial counterparts.2,6,12,17,18,27,28 Two hypotheses have been suggested to explain this apparent discrepancy. First, deep venous drainage may provide a unique vascular flow pattern for brain-stem lesions that somehow increases their risk of hemorrhage.14 Second, and arguably more plausible, the eloquence of the brain stem may increase the detection of minor hemorrhagic events because symptomatic manifestations occur earlier and are more profound than those involving less eloquent areas of the brain. Re-hemorrhage of a brain-stem CM often increases the rate and severity of neurologic deficits and, occasionally, causes death.11,14,17,29,30 In a study from our institution, 48% of nonoperative patients followed a mean of 35 months were worse neurologically at follow-up compared with their presentation.18 Similarly, Porter et al. demonstrated that 50% of patients with brain-stem CMs managed conservatively developed devastating and persistent deficits after re-hemorrhage.17 That these lesions were initially managed nonoperatively suggests that the location is exquisitely eloquent, which may skew the rate of subsequent neurologic decline upward. Nonetheless, it is reasonable to conclude that re-hemorrhage of a brain-stem CMs is associated with a significant risk for worsened neurologic status. Multiple hemorrhages from a CM may occur in temporal proximity.20,31 In a series of 96 patients, Wang et al. found that 46.4% of brain-stem CMs that bled more than once re-hemorrhaged within 6 months; 46.4% re-hemorrhaged between 7 months and 4 years later; and the remaining 7.2% re-hemorrhaged after 4 years.20 Considering CMs of the entire CNS, Barker et al. found that the risk of re-hemorrhage in 63 patients with multiple hemorrhagic episodes was 2% per month for the first 2 years and then decreased to 0.8% per month thereafter.31 This potentially increased likelihood of re-hemorrhage soon after an initial hemorrhage is an important consideration for the timing of surgical intervention. Not surprisingly given the eloquence of the region, 81 to 100% of brain-stem CMs that reach a neurosurgical service will be associated with significant clinical manifestations.11,14–20 The presenting signs and symptoms are caused by the mass effect exerted by the lesion on adjacent neuroanatomic structures. Symptoms can be exacerbated acutely by a hemorrhagic episode or can arise insidiously by slow growth of the CM. Patients often complain of similar past episodes that improved or resolved in the interim. In fact, patients with brain-stem CMs have been diagnosed with multiple sclerosis because of their waxing and waning course.18,32 The nature of presenting signs depends on the location of the CM within the brain stem. Based on large series, 14 to 32% of lesions are located in the mesencephalon, 49 to 64% in the pons, 7 to 21% in the medulla, and the remainder in the pontomesencephalic or pontomedullary junction.14–16,19,20 In a series of 100 patients from our institution, multiple presenting signs were common and included cranial nerve deficits in 69%, ataxia or dysmetria in 43%, sensory deficits in 39%, motor deficits in 38%, speech difficulty in 12%, and an altered level of consciousness in 6%.18 The most common subjective symptoms were headache in 36%, vertigo or dizziness in 24%, nausea and vomiting in 16%, and trigeminal neuralgia in 4%. Of the symptomatic patients, 38% were deteriorating at presentation, whereas the remaining 62% were stable or improving. With the advent of MRI, the radiographic diagnosis of brainstem CMs has improved significantly. Angiography is nondiagnostic for CMs and only occasionally demonstrates a hint of capillary blush or early venous filling.33–35 Although computed tomography (CT) is more useful than angiography, it is nonspecific for detecting CMs.35,36 Hahn et al. reviewed 1361 cases of CMs of the CNS reported between 1854 and 1997. Of these 1361 cases, 1028 (76.6%) were published after 1984 when the use of MRI was becoming widespread.36a Their analysis illustrated the utility of MRI in diagnosing brain-stem CMs. Therefore, surgeons must understand the MRI appearance of CMs including the differences on specific MRI sequences. On MRI, the typical appearance of CMs reflects the presence of hemosiderin and methemoglobin (Fig. 11-1).34 T2-weighted sequences show a central core of mixed signal intensity with a surrounding rim of hypointensity caused by the peripheral deposition of hemosiderin. Smaller lesions show less central heterogeneity and may appear homogenously hypointense. Gradient-echo (GRE) MRI is also useful for diagnosis because it is exquisitely sensitive to hemosiderin; it shows profound hypointensity surrounding the CM. Although T2-weighted and GRE sequences are well suited for diagnosis, both create hemosiderin-related bloom artifact that can obscure the relationship between the CM and surrounding parenchyma.19 Consequently, T1-weighted imaging, with its more detailed anatomic resolution, should be used for surgical planning.
Brain Stem Cavernous Malformations
 Epidemiology and Natural History
Epidemiology and Natural History
Age and Sex Predilection
Hemorrhage and Re-hemorrhage Rates
Outcome after Hemorrhage
Hemorrhage Clustering
 Clinical Presentation
Clinical Presentation
 Diagnostic Studies
Diagnostic Studies
Brain Stem Cavernous Malformations
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree




