Brain Stimulation Methods
 30.1 Electroconvulsive Therapy
30.1 Electroconvulsive Therapy
Convulsive therapies for major psychiatric illnesses predate the modern therapeutic era, with the use of camphor reported as early as the 16th century and the existence of several accounts of camphor convulsive therapies from the late 1700s to the mid-1800s.
Unaware of the history of camphor convulsive therapy, the Hungarian neuropsychiatrist Ladislas von Meduna made the observation that the brains of epileptics had greater than normal numbers of glial cells, whereas those of schizophrenics had fewer, and he hypothesized that there might be a biological antagonism between convulsions and schizophrenia. Following animal experimentation, camphor was (again) selected as the appropriate agent to use for the therapeutic induction of seizures. In 1934, the first catatonic psychotic patient was successfully treated using intramuscular injections of camphor in oil to produce therapeutic seizures. Lucio Bini and Ugo Cerletti were interested in the use of electricity to induce seizures, and, after a series of animal experiments and observation of the use of electricity commercially, they were able to safely apply current across the heads of animals for this purpose. In 1938, the first electroconvulsive treatment (ECT) course was administered to a delusional and incoherent patient, who improved with one treatment and remitted after 11 treatments. Electrical induction of convulsive therapy could be made more reliable and shorter acting than chemically induced convulsive therapies, and, by the early 1940s, it had replaced them. In 1940, the first use of ECT occurred in the United States.
In an effort to reduce the retrograde memory problems that persisted for some patients after the initial recovery period post-ECT, explorations of nondominant electrode placement and alternative, more efficient waveforms were undertaken in subsequent decades. The practice of ECT also benefited from the introduction of controlled trials methodology, which demonstrated its safety and efficacy, and from refinements made in diagnostic systems and the process of informed consent. In the 1980s and 1990s efforts to ensure uniformly high standards of practice were under way with the publication of recommendations for treatment delivery, education, and training by professional organizations in the United States, England, Scandinavia, and Canada, among others.
With the widespread use of pharmacological agents as first-line treatments for major psychiatric disorders, ECT is now more commonly used for patients with resistance to those treatments, except in the case of life-threatening illness due to inanition, severe suicidal symptoms, or catatonia. Although the failure of subconvulsive stimulation to induce the remission of psychiatric illness and the effectiveness of chemical convulsive therapy suggested that the seizure was necessary and sufficient for therapeutic benefit with ECT, it is now known that there is a dose–response relationship with right unilateral ECT and that bilateral ECT is likely to be ineffective with ultrabrief pulse widths. Work continues to explore the underlying mechanisms and biological characteristics of effective ECT treatments, with interest in having the treatment focus on appropriate neural networks with a more efficient stimulus as a method of reducing cognitive side effects. With the growing understanding that depression is a chronic disease for many patients, more emphasis has been placed on continuation and maintenance treatments following an acute course of ECT. Utilization of ECT has diminished since the middle of the 20th century; but because ECT remains the most effective treatment for major depression and a rapidly effective treatment for life-threatening psychiatric conditions, ECT, unlike its contemporaneous somatic therapies, such as insulin coma, remains in the active treatment portfolio of modern therapeutics. Its use has shifted from public to private institutions, and it is estimated that approximately 100,000 patients have received ECT annually over the past few decades in the United States (Table 30.1-1).
 Table 30.1-1
Table 30.1-1
Milestones in the History of Convulsive Therapy

The Nobel Laureate Paul Greengard has suggested that the term electrocortical therapy might be used to replace the current term electroconvulsive therapy. Greengard has acknowledged that if the mechanism of action of ECT, as yet unknown, turns out to be subcortical, then the term might have limited use. Until that time, however, the authors of this text think Greengard’s suggestion deserves consideration. It would help diminish the fear associated with the word convulsion and help destigmatize a very effective treatment method.
ELECTROPHYSIOLOGY IN ELECTROCONVULSIVE THERAPY
Neurons maintain a resting potential across the plasma membrane and may propagate an action potential, which is a transient reversal of the membrane potential. Normal brain activity is desynchronized; that is, neurons fire action potentials asynchronously. A convulsion, or seizure, occurs when a large percentage of neurons fire in unison. Such rhythmical changes in the extracellular potential entrain neighboring neurons, propagate the seizure activity across the cortex and into deeper structures, and eventually engulf the entire brain in high-voltage synchronous neuronal firing. Cellular mechanisms work to contain the seizure activity and to maintain cellular homeostasis, and the seizure eventually ends. In epilepsy, any of possibly several hundred genetic defects can alter the balance in favor of unrestrained activity. In ECT, seizures are triggered in normal neurons by application through the scalp of pulses of current, under conditions that are carefully controlled to create a seizure of a particular duration over the entire brain.
The qualities of the electricity used in ECT can be described by Ohm’s law: E = IR, or I = E/R, in which E is voltage, I is current, and R is resistance. The intensity or dose of electricity in ECT is measured in terms of charge (milliampere-seconds or millicoulombs) or energy (watt-seconds or joules). Resistance is synonymous with impedance and, in the case of ECT, both the electrode’s contact with the body and the nature of the bodily tissues are the major determinants of resistance. The skull has a high impedance; the brain has a low impedance. Because scalp tissues are much better conductors of electricity than bone, only about 20 percent of the applied charge actually enters the skull to excite neurons. The ECT machines that are now widely used can be adjusted to administer the electricity under conditions of constant current, voltage, or energy.
MECHANISM OF ACTION
The induction of a bilateral generalized seizure is necessary for both the beneficial and the adverse effects of ECT. Although a seizure superficially seems as though it is an all-or-none event, some data indicate that not all generalized seizures involve all the neurons in deep brain structures (e.g., the basal ganglia and the thalamus); recruitment of these deep neurons may be necessary for full therapeutic benefit. After the generalized seizure, the electroencephalogram (EEG) shows about 60 to 90 seconds of postictal suppression. This period is followed by the appearance of high-voltage delta and theta waves and a return of the EEG to preseizure appearance in about 30 minutes. During the course of a series of ECT treatments, the interictal EEG is generally slower and of greater amplitude than usual, but the EEG returns to pretreatment appearance 1 month to 1 year after the end of the course of treatment.
One research approach to the mechanism of action for ECT has been to study the neurophysiological effects of treatment. Positron emission tomography (PET) studies of both cerebral blood flow and glucose use have shown that, during seizures, cerebral blood flow, use of glucose and oxygen, and permeability of the blood–brain barrier increase. After the seizure, blood flow and glucose metabolism are decreased, perhaps most markedly in the frontal lobes. Some research indicates that the degree of decrease in cerebral metabolism is correlated with therapeutic response.
Seizure foci in idiopathic epilepsy are hypometabolic during interictal periods; ECT itself acts as an anticonvulsant because its administration is associated with an increase in the seizure threshold as treatment progresses. Recent data suggest that for 1 to 2 months following a session of ECT, EEGs record a large increase in slow-wave activity located over the prefrontal cortex in patients who responded well to the ECT. High-intensity, bilateral stimulation produced the best response; low-intensity, unilateral stimulation, the weakest. These data are of unclear significance, however, because the specific EEG correlate disappeared 2 months after ECT, whereas the clinical benefit persisted.
ECT affects the cellular mechanisms of memory and mood regulation and raises the seizure threshold. The latter effect may be blocked by the opiate antagonist naloxone (Narcan).
Neurochemical research into the mechanisms of action of ECT has focused on changes in neurotransmitter receptors and, recently, changes in second-messenger systems. Virtually every neurotransmitter system is affected by ECT, but a series of ECT sessions results in downregulation of postsynaptic β-adrenergic receptors, the same receptor change observed with virtually all antidepressant treatments. The effects of ECT on serotonergic neurons remain controversial. Various research studies have reported an increase in postsynaptic serotonin receptors, no change in serotonin receptors, and a change in the presynaptic regulation of serotonin release. ECT has also been reported to effect changes in the muscarinic, cholinergic, and dopaminergic neuronal systems. In second-messenger systems, ECT has been reported to affect the coupling of G-proteins to receptors, the activity of adenylyl cyclase and phospholipase C, and the regulation of calcium entry into neurons.
Recently, there has been increased interest in structural changes in the brain associated with psychiatric syndromes and response to treatment. This has been particularly so for microscopic changes associated with electroconvulsive stimulation, as well as antidepressant and other medications. In animals, mostly rodents, synaptic plasticity in hippocampus, including mossy fiber sprouting, alterations in cytoskeletal structure, increased connectivity in perforant pathways, promotion of neurogenesis, and suppression of apoptosis have been observed. Many of these structural events are also observed, although to a lesser extent, with antidepressant medications such as fluoxetine (Prozac). These reports have also galvanized controversy over various aspects of the technical validity of the observations. It is unknown whether such changes occur clinically and, if they do, what significance to efficacy and cognitive side effects might be discovered.
INDICATIONS
Major Depressive Disorder
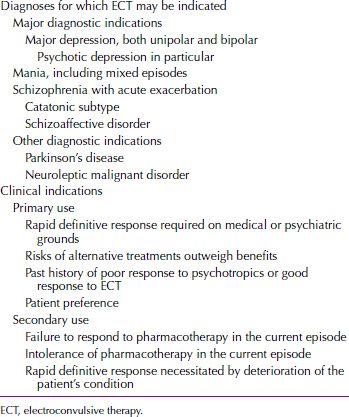
The most common indication for ECT is major depressive disorder, for which ECT is the fastest and most effective available therapy. ECT should be considered for use in patients who have failed medication trials, have not tolerated medications, have severe or psychotic symptoms, are acutely suicidal or homicidal, or have marked symptoms of agitation or stupor. Controlled studies have shown that up to 70 percent of patients who fail to respond to antidepressant medications may respond positively to ECT. Table 30.1-2 presents the indications for the use of ECT.
 Table 30.1-2
Table 30.1-2
Indications for the Use of Electroconvulsive Therapy
ECT is effective for depression in both major depressive disorder and bipolar I disorder. Delusional or psychotic depression has long been considered particularly responsive to ECT; but recent studies have indicated that major depressive episodes with psychotic features are no more responsive to ECT than nonpsychotic depressive disorders. Nevertheless, because major depressive episodes with psychotic features respond poorly to antidepressant pharmacotherapy alone, ECT should be considered much more often as the first-line treatment for patients with the disorder. Major depressive disorder with melancholic features (e.g., markedly severe symptoms, psychomotor retardation, early morning awakening, diurnal variation, decreased appetite and weight, and agitation) is considered likely to respond to ECT. ECT is particularly indicated for persons who are severely depressed, who have psychotic symptoms, who show suicidal intent, or who refuse to eat. Depressed patients less likely to respond to ECT include those with somatization disorder. Elderly patients tend to respond to ECT more slowly than do young patients. ECT is a treatment for major depressive episode and does not provide prophylaxis unless it is administered on a long-term maintenance basis.
Manic Episodes
ECT is at least equal to lithium (Eskalith) in the treatment of acute manic episodes. The pharmacological treatment of manic episodes, however, is so effective in the short term and for prophylaxis that the use of ECT to treat manic episodes is generally limited to situations with specific contraindications to all available pharmacological approaches. The relative rapidity of the ECT response indicates its usefulness for patients whose manic behavior has produced dangerous levels of exhaustion. ECT should not be used for a patient who is receiving lithium, because lithium can lower the seizure threshold and cause a prolonged seizure.
Schizophrenia
Although an effective treatment for the symptoms of acute schizophrenia, ECT is not for those of chronic schizophrenia. Patients with schizophrenia who have marked positive symptoms, catatonia, or affective symptoms are considered most likely to respond to ECT. In such patients, the efficacy of ECT is about equal to that of antipsychotics, but improvement may occur faster.
Other Indications
Small studies have found ECT effective in the treatment of catatonia, a symptom associated with mood disorders, schizophrenia, and medical and neurological disorders. ECT is also reportedly useful to treat episodic psychoses, atypical psychoses, obsessive-compulsive disorder, and delirium and such medical conditions as neuroleptic malignant syndrome, hypopituitarism, intractable seizure disorders, and the on–off phenomenon of Parkinson’s disease. ECT may also be the treatment of choice for depressed suicidal pregnant women who require treatment and cannot take medication; for geriatric and medically ill patients who cannot take antidepressant drugs safely; and perhaps even for severely depressed and suicidal children and adolescents who may be less likely to respond to antidepressant drugs than are adults. ECT is not effective in somatization disorder (unless accompanied by depression), personality disorders, and anxiety disorders.
CLINICAL GUIDELINES
Patients and their families are often apprehensive about ECT; therefore, clinicians must explain both beneficial and adverse effects and alternative treatment approaches. The informed-consent process should be documented in the patients’ medical records and should include a discussion of the disorder, its natural course, and the option of receiving no treatment. Printed literature and videotapes about ECT may be useful in attempting to obtain a truly informed consent. The use of involuntary ECT is rare today and should be reserved for patients who urgently need treatment and who have a legally appointed guardian who has agreed to its use. Clinicians must know local, state, and federal laws about the use of ECT.
Pretreatment Evaluation
Pretreatment evaluation should include standard physical, neurological, and preanesthesia examinations and a complete medical history. Laboratory evaluations should include blood and urine chemistries, a chest X-ray, and an electrocardiogram (ECG). A dental examination to assess the state of patients’ dentition is advisable for elderly patients and patients who have had inadequate dental care. An X-ray of the spine is needed if other evidence of a spinal disorder is seen. Computed tomography (CT) or magnetic resonance imaging (MRI) should be performed if a clinician suspects the presence of a seizure disorder or a space-occupying lesion. Practitioners of ECT no longer consider even a space-occupying lesion to be an absolute contraindication to ECT, but with such patients the procedure should be performed only by experts.
Concomitant Medications. Patients’ ongoing medications should be assessed for possible interactions with the induction of a seizure, for effects (both positive and negative) on the seizure threshold, and for drug interactions with the medications used during ECT. The use of tricyclic and tetracyclic drugs, monoamine oxidase inhibitors, and antipsychotics is generally considered acceptable. Benzodiazepines used for anxiety should be withdrawn because of their anticonvulsant activity; lithium should be withdrawn because it can result in increased postictal delirium and can prolong seizure activity; clozapine (Clozaril) and bupropion (Wellbutrin) should be withdrawn because they are associated with the development of late-appearing seizures. Lidocaine (Xylocaine) should not be administered during ECT because it markedly increases the seizure threshold; theophylline (Theo-Dur) is contraindicated because it increases the duration of seizures. Reserpine (Serpasil) is also contraindicated because it is associated with further compromise of the respiratory and cardiovascular systems during ECT.
Premedications, Anesthetics, and Muscle Relaxants
Patients should not be given anything orally for 6 hours before treatment. Just before the procedure, the patient’s mouth should be checked for dentures and other foreign objects, and an intravenous (IV) line should be established. A bite block is inserted in the mouth just before the treatment is administered to protect the patient’s teeth and tongue during the seizure. Except for the brief interval of electrical stimulation, 100 percent oxygen is administered at a rate of 5 L a minute during the procedure until spontaneous respiration returns. Emergency equipment for establishing an airway should be immediately available in case it is needed.
Muscarinic Anticholinergic Drugs. Muscarinic anticholinergic drugs are administered before ECT to minimize oral and respiratory secretions and to block bradycardias and asystoles, unless the resting heart rate is above 90 beats a minute. Some ECT centers have stopped the routine use of anticholinergics as premedications, although their use is still indicated for patients taking β-adrenergic receptor antagonists and those with ventricular ectopic beats. The most commonly used drug is atropine, which can be administered 0.3 to 0.6 mg intramuscularly (IM) or subcutaneously (SC) 30 to 60 minutes before the anesthetic or 0.4 to 1.0 mg IV 2 or 3 minutes before the anesthetic. An option is to use glycopyrrolate (Robinul) (0.2 to 0.4 mg IM, IV, or SC), which is less likely to cross the blood–brain barrier and less likely to cause cognitive dysfunction and nausea, although it is thought to have less cardiovascular protective activity than does atropine.
Anesthesia. Administration of ECT requires general anesthesia and oxygenation. The depth of anesthesia should be as light as possible, not only to minimize adverse effects but also to avoid elevating the seizure threshold associated with many anesthetics. Methohexital (Brevital) (0.75 to 1.0 mg/kg IV bolus) is the most commonly used anesthetic because of its shorter duration of action and lower association with postictal arrhythmias than thiopental (Pentothal) (usual dose 2 to 3 mg/kg IV), although this difference in cardiac effects is not universally accepted. Four other anesthetic alternatives are etomidate (Amidate), ketamine (Ketalar), alfentanil (Alfenta), and propofol (Diprivan). Etomidate (0.15 to 0.3 mg/kg IV) is sometimes used because it does not increase the seizure threshold; this effect is particularly useful for elderly patients because the seizure threshold increases with age. Ketamine (6 to 10 mg/kg IM) is sometimes used because it does not increase the seizure threshold, although its use is limited by the frequent association of psychotic symptoms with emergence from anesthesia with this drug. Alfentanil (2 to 9 mg/kg IV) is sometimes coadministered with barbiturates to allow the use of low doses of the barbiturate anesthetics and, thus, reduce the seizure threshold less than usual, although its use can be associated with an increased incidence of nausea. Propofol (0.5 to 3.5 mg/kg IV) is less useful because of its strong anticonvulsant properties.
Muscle Relaxants. After the onset of the anesthetic effect, usually within a minute, a muscle relaxant is administered to minimize the risk of bone fractures and other injuries resulting from motor activity during the seizure. The goal is to produce profound relaxation of the muscles, not necessarily to paralyze them, unless the patient has a history of osteoporosis or spinal injury or has a pacemaker and, therefore, is at risk for injury related to motor activity during the seizure. Succinylcholine (Anectine), an ultrafast-acting depolarizing blocking agent, has gained virtually universal acceptance for the purpose. Succinylcholine is usually administered in a dose of 0.5 to 1 mg/kg as an IV bolus or drip. Because succinylcholine is a depolarizing agent, its action is marked by the presence of muscle fasciculations, which move in a rostrocaudal progression. The disappearance of these movements in the feet or the absence of muscle contractions after peripheral nerve stimulation indicates maximal muscle relaxation. In some patients, tubocurarine (3 mg IV) is administered to prevent myoclonus and increases in potassium and muscle enzymes; these reactions can be a problem in patients with musculoskeletal or cardiac disease. To monitor the duration of the convulsion, a blood pressure cuff may be inflated at the ankle to a pressure in excess of the systolic pressure before infusion of the muscle relaxant to allow observation of relatively innocuous seizure activity in the foot muscles.
If a patient has a known history of pseudocholinesterase deficiency, atracurium (Tracrium) (0.5 to 1 mg/kg IV) or curare can be used instead of succinylcholine. In such a patient, the metabolism of succinylcholine is disrupted, and prolonged apnea may necessitate emergency airway management. In general, however, because of the short half-life of succinylcholine, the duration of apnea after its administration is generally shorter than the delay in regaining consciousness caused by the anesthetic and the postictal state.
Electrode Placement. Historically, most practitioners have used bifrontotemporal electrode placement because of its reliability in producing efficacy and its ease of use. This electrode placement is also associated with more short-term and long-term adverse cognitive effects and is more likely to produce delirium, which may require interrupting a course of ECT and perhaps even terminating it before optimal therapeutic effects have been obtained. Hence, when bifrontotemporal ECT is used, attention should be paid to restricting the dose to a moderately suprathreshold level to attenuate adverse cognitive effects as much as possible. It should be emphasized that the combination of ultrabrief pulse and bifrontotemporal electrode placement has not been demonstrated to be effective. Treatment with bilateral electrode placements, particularly a bifrontal configuration, is more likely to manifest EEG seizure without motor seizure, and EEG monitoring can be particularly useful in detecting its occurrence.
Newer electrode placements include bifrontal configuration and asymmetrical placements. There are limitations to these strategies, imposed by the fact that the high impedance of the skull and scalp causes spreading of the electrical stimulus and restricts possibilities for localization of the stimulus. Bifrontal electrode placement, with positioning far enough laterally to minimize interference with impedance relations, has been investigated, and there have been several demonstrations that bifrontal electrode placements are equally effective to bifrontotemporal and adequately dosed right unilateral electrode configurations. Evidence of advantages in sparing of cognitive effects is quite preliminary, and adequately powered investigations with more extensive and sensitive cognitive batteries are needed. Seizure threshold is likely to be relatively higher with bifrontal ECT.
The relatively better cognitive side effect profile of right unilateral ECT should encourage wider use now that the efficacy of this electrode placement can be ensured with adequate dosing strategies. In contrast to bilateral ECT, a dose closer to 500 percent above the seizure threshold is more likely to ensure efficacy. ECT devices in the United States are restricted to an output in the range of 504 to 576 mCi. Approximately 90 percent of patients have seizure thresholds that can accommodate optimal dosing with brief-pulse right unilateral ECT, and the combination of right unilateral electrode placement with ultrabrief pulse width extends the range of US devices so that most patients can be treated within these constraints. Individuals with an exceptionally high seizure threshold may require bilateral electrode placements to remain within the device restrictions. Maximizing interelectrode distance by using the d’Elia placement may also be optimal. Many other right unilateral placements have been described, but there is little work to support their use (Fig. 30.1-1).
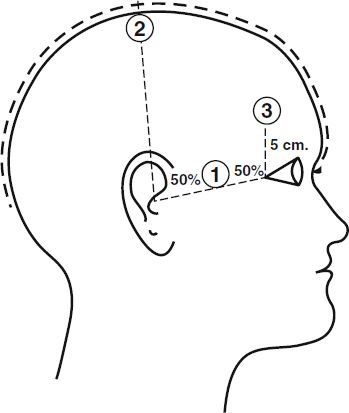
FIGURE 30.1-1
Electrode placements. Position 1 represents the frontotemporal position, used for both electrodes, one on each side of the head, in conducting bilateral electroconvulsive therapy (ECT). For right unilateral ECT, one electrode is in the right frontotemporal position, and the other is just to the right of the vertex at position 2. (Courtesy of American Psychiatric Association, with permission.)
There has been some concern that left-handed patients may require different electrode placement than right-handed patients, especially if unilateral placement is desired. Even when handedness is lateralized to the left, the anatomic localization of language function in 70 percent of left-handed individuals is the same as in those who are right-handed. Furthermore, there is evidence for independent lateralization of affect, with the right hemisphere involved in sustaining depressed mood regardless of handedness. Because of limited indications that affective function and efficacy of ECT are associated with handedness, handedness is not generally used to guide the choice of electrode placement.
Electrical Stimulus
The electrical stimulus must be sufficiently strong to reach the seizure threshold (the level of intensity needed to produce a seizure). The electrical stimulus is given in cycles, and each cycle contains a positive and a negative wave. Old machines use a sine wave; however, this type of machine is now considered obsolete because of the inefficiency of that wave shape. When a sine wave is delivered, the electrical stimulus in the sine wave before the seizure threshold is reached and after the seizure is activated is unnecessary and excessive. Modern ECT machines use a brief pulse waveform that administers the electrical stimulus usually in 1 to 2 milliseconds at a rate of 30 to 100 pulses a second. Machines that use an ultrabrief pulse (0.5 milliseconds) are not as effective as brief pulse machines.
Establishing a patient’s seizure threshold is not straightforward. A 40 times variability in seizure thresholds occurs among patients. In addition, during the course of ECT treatment, a patient’s seizure threshold may increase 25 to 200 percent. The seizure threshold is also higher in men than in women and higher in older than in younger adults. A common technique is to initiate treatment at an electrical stimulus that is thought to be below the seizure threshold for a particular patient and then to increase this intensity by 100 percent for unilateral placement and by 50 percent for bilateral placement until the seizure threshold is reached. A debate in the literature concerns whether a minimally suprathreshold dose, a moderately suprathreshold dose (one and a half times the threshold), or a high suprathreshold dose (three times the threshold) is preferable. The debate about stimulus intensity resembles the debate about electrode placement. Essentially, the data support the conclusion that doses of three times the threshold are the most rapidly effective and that minimal suprathreshold doses are associated with the fewest and least severe cognitive adverse effects.
Induced Seizures
A brief muscular contraction, usually strongest in a patient’s jaw and facial muscles, is seen concurrently with the flow of stimulus current, regardless of whether a seizure occurs. The first behavioral sign of the seizure is often a plantar extension, which lasts 10 to 20 seconds and marks the tonic phase. This phase is followed by rhythmic (i.e., clonic) contractions that decrease in frequency and finally disappear. The tonic phase is marked by high-frequency, sharp EEG activity on which a higher frequency muscle artifact may be superimposed. During the clonic phase, bursts of polyspike activity occur simultaneously with the muscular contractions but usually persist for at least a few seconds after the clonic movements stop.
Monitoring Seizures. A physician must have an objective measure that a bilateral generalized seizure has occurred after the stimulation. The physician should be able to observe either some evidence of tonic-clonic movements or electrophysiological evidence of seizure activity from the EEG or electromyogram (EMG). Seizures with unilateral ECT are asymmetrical, with higher ictal EEG amplitudes over the stimulated hemisphere than over the nonstimulated hemisphere. Occasionally, unilateral seizures are induced; for this reason, at least a single pair of EEG electrodes should be placed over the contralateral hemisphere when using unilateral ECT. For a seizure to be effective in the course of ECT, it should last at least 25 seconds.
Failure to Induce Seizures. If a particular stimulus fails to cause a seizure of sufficient duration, up to four attempts at seizure induction can be tried during a course of treatment. The onset of seizure activity is sometimes delayed as long as 20 to 40 seconds after the stimulus administration. If a stimulus fails to result in a seizure, the contact between the electrodes and the skin should be checked, and the intensity of the stimulus should be increased by 25 to 100 percent. The clinician can also change the anesthetic agent to minimize increases in the seizure threshold caused by the anesthetic. Additional procedures to lower the seizure threshold include hyperventilation and administration of 500 to 2,000 mg IV of caffeine sodium benzoate 5 to 10 minutes before the stimulus.
Prolonged and Tardive Seizures. Prolonged seizures (seizures lasting more than 180 seconds) and status epilepticus can be terminated either with additional doses of the barbiturate anesthetic agent or with IV diazepam (Valium) (5 to 10 mg). Management of such complications should be accompanied by intubation, because the oral airway is insufficient to maintain adequate ventilation over an extended apneic period. Tardive seizures—that is, additional seizures appearing some time after the ECT treatment—may develop in patients with preexisting seizure disorders. Rarely, ECT precipitates the development of an epileptic disorder in patients. Such situations should be managed clinically as if they were pure epileptic disorders.
Number and Spacing of Treatments
ECT treatments are usually administered two to three times a week; twice-weekly treatments are associated with less memory impairment than thrice-weekly treatments. In general, the course of treatment of major depressive disorder can take 6 to 12 treatments (although up to 20 sessions are possible); the treatment of manic episodes can take 8 to 20 treatments; the treatment of schizophrenia can take more than 15 treatments; and the treatment of catatonia and delirium can take as few as 1 to 4 treatments. Treatment should continue until the patient achieves what is considered the maximal therapeutic response. Further treatment does not yield any therapeutic benefit, but increases the severity and duration of the adverse effects. The point of maximal improvement is usually thought to occur when a patient fails to continue to improve after two consecutive treatments. If a patient is not improving after 6 to 10 sessions, bilateral placement and high-density treatment (three times the seizure threshold) should be attempted before ECT is abandoned.
Multiple-Monitored Electroconvulsive Therapy. Multiple-monitored ECT (MMECT) involves giving multiple ECT stimuli during a single session, most commonly two bilateral stimuli within 2 minutes. This approach may be warranted in severely ill patients and in those at especially high risk from the anesthetic procedures. MMECT is associated with the most frequent occurrences of serious cognitive adverse effects.
Maintenance Treatment
A short-term course of ECT induces a remission in symptoms but does not, of itself, prevent a relapse. Post-ECT maintenance treatment should always be considered. Maintenance therapy is generally pharmacological, but maintenance ECT treatments (weekly, biweekly, or monthly) have been reported to be effective relapse prevention treatments, although data from large studies are lacking. Indications for maintenance ECT treatments can include rapid relapse after initial ECT, severe symptoms, psychotic symptoms, and the inability to tolerate medications. If ECT was used because a patient was unresponsive to a specific medication, then, following ECT, the patient should be given a trial of a different medication.
Failure of Electroconvulsive Therapy Trial
Patients who fail to improve after a trial of ECT should again be treated with the pharmacological agents that failed in the past. Although the data are primarily anecdotal, many reports indicate that patients who had previously failed to improve while taking an antidepressant drug do improve while taking the same drug after receiving a course of ECT treatments, even if the ECT seemed to be a therapeutic failure. Nonetheless, with the increased availability of drugs that act at diverse receptor sites, it is less often necessary to return to a drug that has failed than it was formerly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








